Abstract
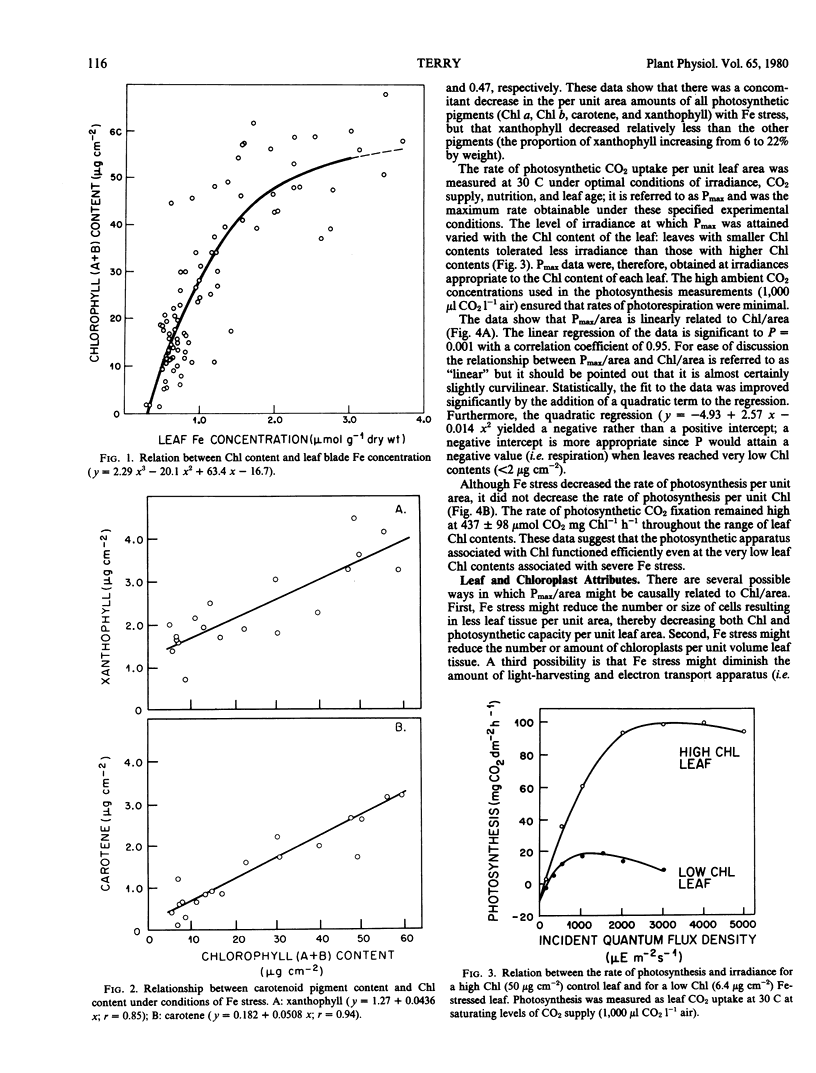
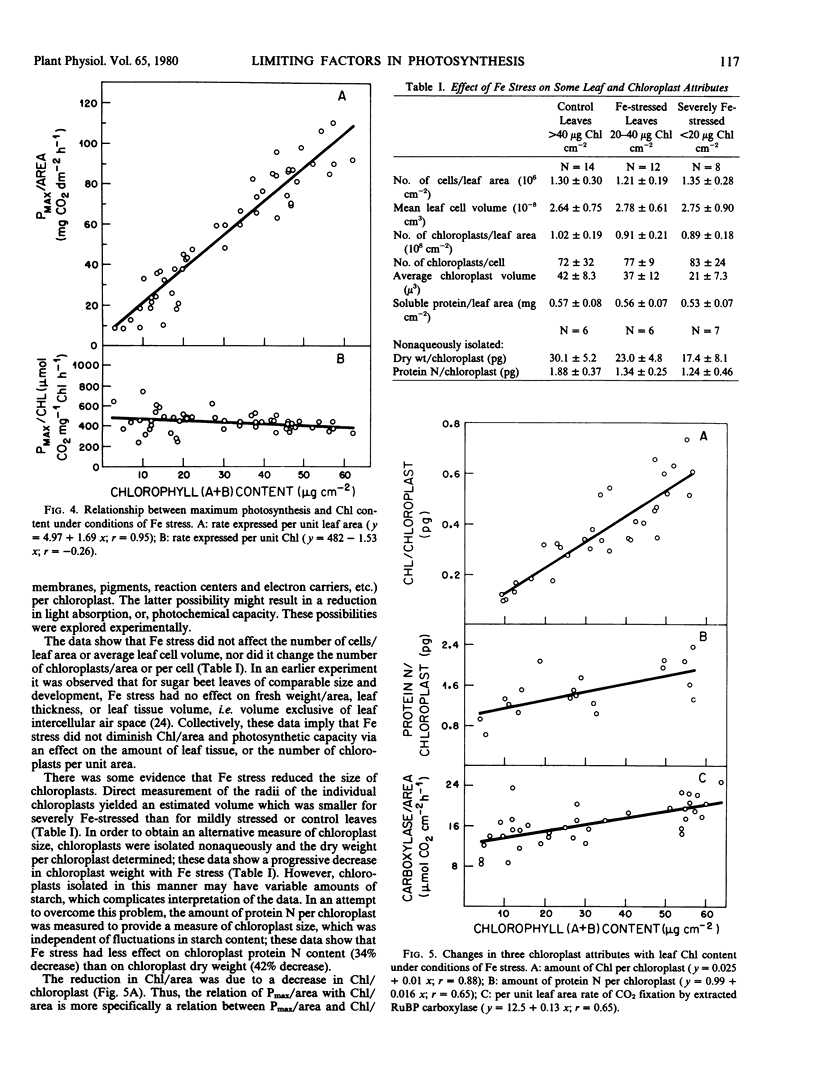
The possibility of using Fe stress as an experimental tool in the study of limiting factors was explored. Results show that Fe stress decreased the chlorophyll (Chl) a, Chl b, carotene, and xanthophyll content of leaves of sugar beets (Beta vulgaris L.) and that the maximum rate of photosynthetic CO2 uptake (Pmax) per unit area was linearly related to Chl (a + b) per unit area. Measurements of noncyclic ATP formation by isolated chloroplasts at light saturation indicate that photosynthetic electron transport capacity decreased concomitantly with pigment content under Fe stress.
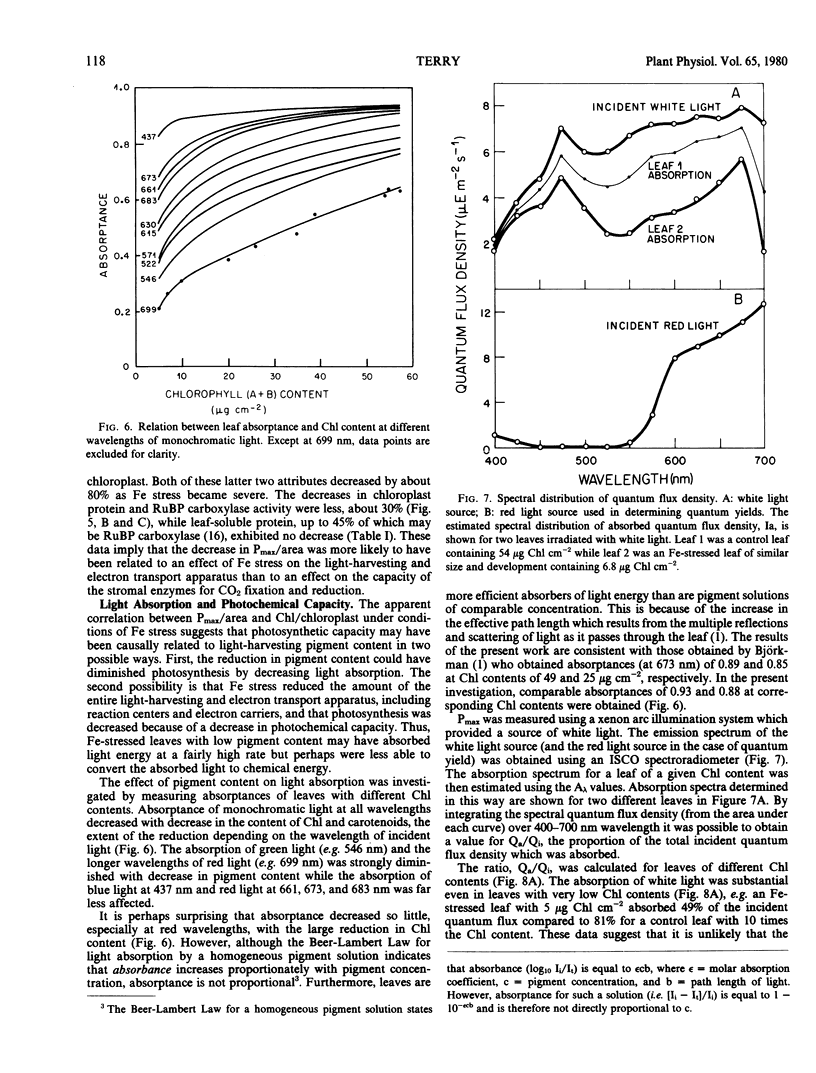
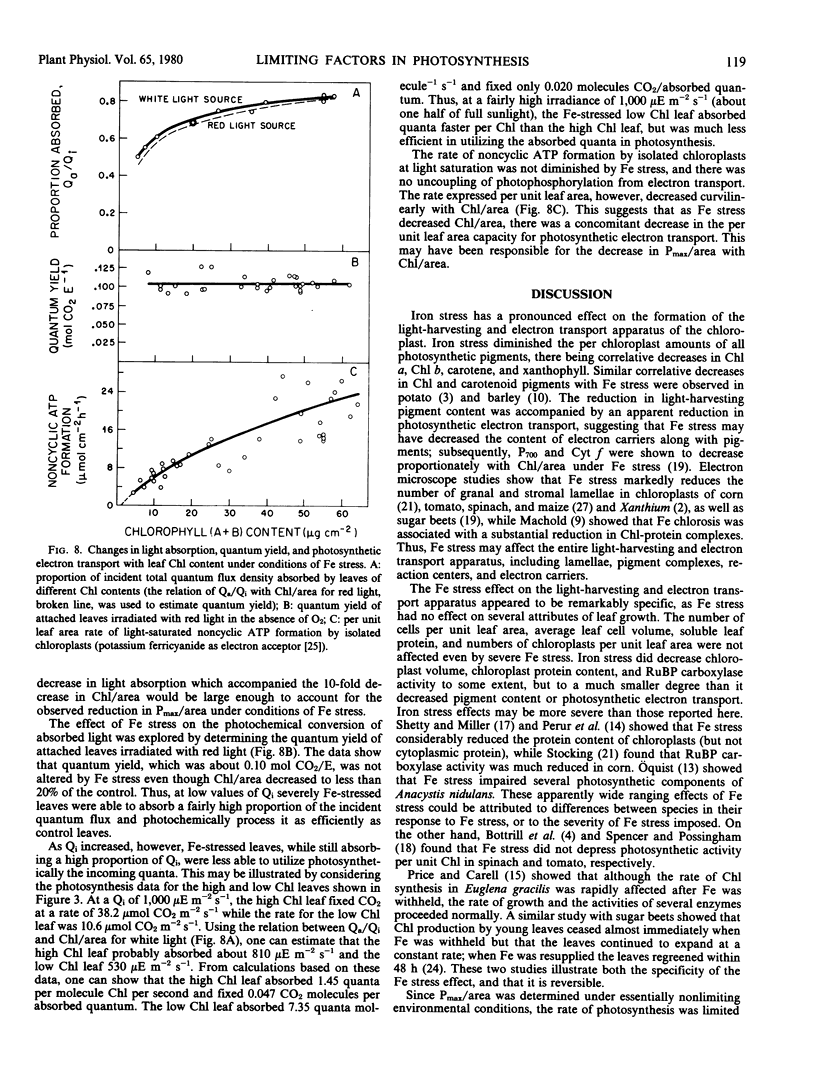
Iron stress decreased Chl per chloroplast but had no effect on the number of leaf cells per unit area, average leaf cell volume, number of chloroplasts per unit area, or leaf soluble protein per unit area. Average chloroplast volume, protein N per chloroplast, and ribulose bisphosphate carboxylase activity were diminished by Fe stress but to a lesser extent than Chl per chloroplast. The reduction in pigment concentration with Fe stress led to a relatively small decrease in light absorption, the fraction of incident light absorbed remaining high (49%) even at very low leaf Chl contents. There was no apparent change in the quantum yield of attached leaves at low irradiances, but at high irradiances, the capacity to convert absorbed light to chemical energy was greatly diminished in Fe-stressed leaves.
The results suggest: (a) that Pmax per unit area are decreased linearly with Chl per unit area because of a decrease in photochemical capacity rather than a change in light absorption; and (b) that the effect of Fe stress may be sufficiently specific for it to be used as an experimental tool for the control and study of photochemical capacity in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Machold O. Lamellar proteins of green and chlorotic chloroplasts as affected by iron deficiency and antibiotics. Biochim Biophys Acta. 1971 May 13;238(2):324–331. doi: 10.1016/0005-2787(71)90099-2. [DOI] [PubMed] [Google Scholar]
- Mackinney G. DEVELOPMENT OF THE CHLOROPHYLL AND CAROTENOID PIGMENTS IN BARLEY SEEDLINGS. Plant Physiol. 1935 Apr;10(2):365–373. doi: 10.1104/pp.10.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwain B. D., Arnon D. I. Enhancement effects and the identity of the two photochemical reactions of photosynthesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):989–996. doi: 10.1073/pnas.61.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles C. D., Daniel D. J. Chloroplast Reactions of Photosynthetic Mutants in Zea mays. Plant Physiol. 1974 Apr;53(4):589–595. doi: 10.1104/pp.53.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perur N. G., Smith R. L., Wiebe H. H. Effect of iron chlorosis on protein fractions of corn leaf tissue. Plant Physiol. 1961 Nov;36(6):736–739. doi: 10.1104/pp.36.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. A., Carell E. F. Control by Iron of Chlorophyll Formation and Growth in Euglena gracilis. Plant Physiol. 1964 Sep;39(5):862–868. doi: 10.1104/pp.39.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty A. S., Miller G. W. Influence of Iron Chlorosis on Pigment and Protein Metabolism in Leaves of Nicotiana tabacum L. Plant Physiol. 1966 Mar;41(3):415–421. doi: 10.1104/pp.41.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller S., Terry N. Limiting Factors in Photosynthesis: II. IRON STRESS DIMINISHES PHOTOCHEMICAL CAPACITY BY REDUCING THE NUMBER OF PHOTOSYNTHETIC UNITS. Plant Physiol. 1980 Jan;65(1):121–125. doi: 10.1104/pp.65.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C. R. Chloroplast Isolation in Nonaqueous Media. Plant Physiol. 1959 Jan;34(1):56–61. doi: 10.1104/pp.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C. R. Iron deficiency and the structure and physiology of maize chloroplasts. Plant Physiol. 1975 Apr;55(4):626–631. doi: 10.1104/pp.55.4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N., Huston R. P. Effects of calcium on the photosynthesis of intact leaves and isolated chloroplasts of sugar beets. Plant Physiol. 1975 May;55(5):923–927. doi: 10.1104/pp.55.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Photosynthesis, growth, and the role of chloride. Plant Physiol. 1977 Jul;60(1):69–75. doi: 10.1104/pp.60.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N., Ulrich A. Effects of potassium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1973 Apr;51(4):783–786. doi: 10.1104/pp.51.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]


