Abstract
Background: Low calcium dialysate with 1.25 mmol/l calcium concentration has been proposed to replace standard calcium dialysate in peritoneal dialysis patients taking calcium-containing phosphate binder to prevent hypercalcaemia. We conducted a meta-analysis to evaluate long term effects on mineral and bone metabolism by low versus standard calcium dialysate in peritoneal dialysis. Method: Clinical studies comparing low versus standard calcium dialysate in peritoneal dialysis patients were identified by searching PubMed (from 1990 to October 2013) and EMBASE (from 1990 to October 2013). Major outcomes extracted for meta-analysis were: serum total and ionized calcium, phosphate, parathyroid hormone and bone metabolism. Statistical analyses were performed using the Review Manager, version 5.1.0 (Cochrane Collaboration, Oxford, UK). Results: Four studies were identified for meta-analysis. A total of 240 peritoneal dialysis patients received standard calcium dialysate and 106 patients were given low calcium dialysate. 1-2 year after peritoneal dialysis, both serum total and ionized calcium were lower in low calcium dialysate patients as compared with standard dialysate patients (Total calcium: MD, 0.09; 95% CI, 0.05 0.13; P < 0.0001; Ionized calcium: MD, 0.04; 95% CI, 0.02 0.06; P < 0.0001). No statistical difference was observed in phosphate level between two groups (MD, -0.05; 95% CI, -0.13 0.02; P = 0.19). Intact parathyroid hormone level was significantly increased in low calcium dialysate patients. No clinically significant long term change of bone metabolism was observed between low and standard calcium dialysate treated patients. Conclusion: Long term (1-2 year) use of low calcium dialysate with 1.25 mmol/l calcium concentration in peritoneal dialysis patients results in decrease of serum total and ionized calcium level and does not change serum phosphate level. No clinical significance in the change of bone metabolism was observed between low and standard calcium dialysate patients despite the increase of serum parathyroid hormone in low calcium dialysate group.
Keywords: Peritoneal dialysis, low-calcium dialysate, renal osteodystrophy, meta-analysis
Introduction
Chronic kidney disease (CKD)-induced systemic abnormal mineral metabolism leads to severe disruption in bone turnover and mineralization [1]. One of the clinical consequences of CKD is hyperphosphatemia, which is an important risk factor of chronic kidney disease-mineral and bone disorder (CKD-MBD) [2]. Peritoneal dialysis (PD) is the major option to maintain the normal mineral and bone metabolism in patients with end-stage of CKD. Whereas a calcium-containing phosphate binder has become the preferred choice to control hyperphosphatemia, hypercalcaemia is a common serious side effect of this treatment in patients dialyzed with standard calcium dialysate (SCD) of 1.75 mmol/l calcium concentration. To prevent hypercalcaemia, low calcium dialysate (LCD) of 1.25 mmol/l calcium concentration has been proposed to replace SCD in PD patients [3]. However, LCD was reported to accelerate secondary hyperparathyroidism and progression of bone disease [4]. Despite the popular use of LCD, there are few studies on its long term effects on bone metabolism. To address this problem, we conducted a meta-analysis from four studies comparing long term (12-24 month) clinical effects on mineral and bone metabolism between SCD and LCD.
Methods
Database and search strategy
We searched the following database for relevant studies: PubMed (from 1990 to October 2013), and EMBASE (from 1990 to October 2013). Our search terms for PubMed are: (“peritoneal dialysis” [MeSH Terms] OR (“peritoneal” [All Fields] AND “dialysis” [All Fields]) OR “peritoneal dialysis” [All Fields]) AND (“bone and bones” [MeSH Terms] OR (“bone” [All Fields] AND “bones” [All Fields]) OR “bone and bones” [All Fields] OR “bone” [All Fields]) AND (“minerals” [MeSH Terms] OR “minerals” [All Fields] OR “mineral” [All Fields]) OR (“metabolism” [Subheading] OR “metabolism” [All Fields] OR “metabolism” [MeSH Terms] OR “metabolism” [All Fields] OR “metabolic networks and pathways” [MeSH Terms] OR (“metabolic” [All Fields] AND “networks” [All Fields] AND “pathways” [All Fields]) OR “metabolic networks and pathways” [All Fields])) AND (low[All Fields] AND (“calcium” [MeSH Terms] OR “calcium” [All Fields])). Search terms for EMBASE are: ‘peritoneal dialysis’/exp or ‘peritoneal dialysis’ and ‘low calcium’ and (‘bone’/exp or ‘bone’) and [1990-2014]/py.
Study selection criteria and quality assessment
Studies comparing clinical effects on mineral and bone metabolism between SCD and LCD in PD patients were selected for meta-analysis. The quality of included trials was assessed using the Jadad scale score (0 to 5), including method of randomization, use of control and description of withdraws and dropouts. A score of 3 or above indicated high quality [5]. XC and JZ independently conducted database search and study selection. Any discrepancy between their results was resolved by group discussion of all participants.
DATA extraction and statistical analysis
Following information was extracted from selected studies: author, publication year, study design, number of subject enrolled, blood total calcium, ionized calcium, phosphate, intact parathyroid hormone (i-PTH) and a variety of data on bone metabolism. Extracted data on mineral metabolism that were presented as mean ± standard deviation (SD) were pooled and analyzed using the Review Manager, version 5.1.0 (Cochrane Collaboration, Oxford, UK). Extracted data that were presented as mean ± standard error (SEM) were converted to mean ± SD using the same software. Continuous outcomes were presented as a mean difference (MD) with a 95% confidence interval (CI). The presence of heterogeneity across trials was also evaluated. A P value < 0.10 was considered statistically significant. Random-effects models were used for the meta-analysis in case heterogeneity was statistically significant; otherwise, fixed-effects models were applied. Extracted data that could not be pooled for meta-analysis were qualitatively summarized in tables.
Results
Study identification and characteristics
A total of 108 unique records were identified by our search strategy. After initial screening by title and abstract, 5 relevant studies were selected for full text retrieving. One study was excluded as no full text was available. Remaining 4 studies were selected for data synthesis and meta-analysis [6-9]. (Figure 1) Among these studies, a total of 240 PD patients received SCD and 106 patients were given LCD. Jadad score for Kang [6] is 1 as it was not a randomized trial. Other three studies were all randomized trials with Jadad score range from 3 to 5, indicating high quality of these studies. Study characteristics were summarized in Table 1.
Figure 1.
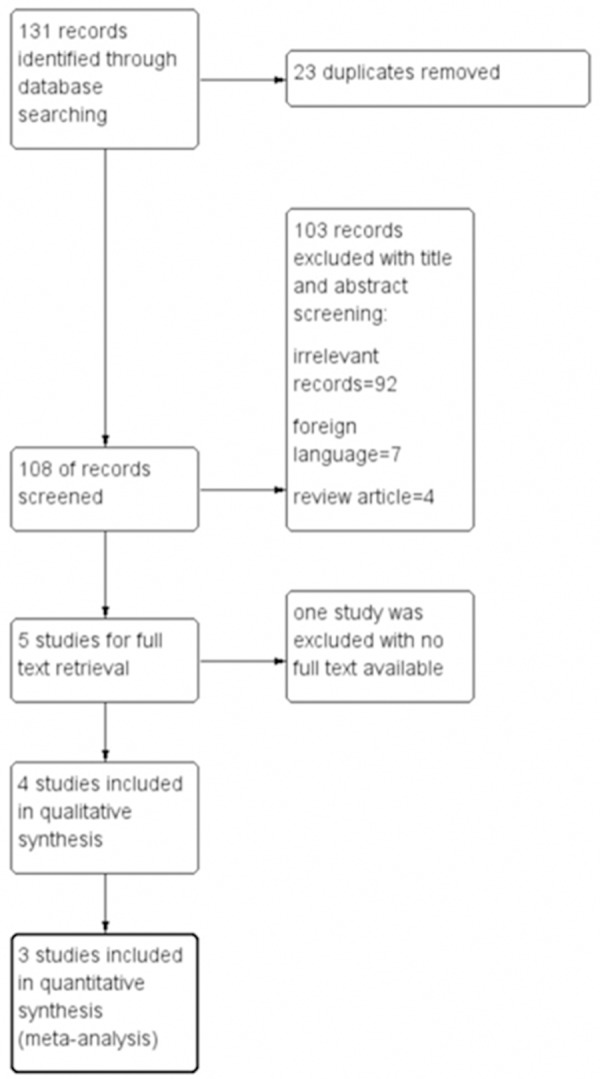
Flow diagram of study selection.
Table 1.
Study characteristics and quality assessment
| Study (year) | Study design | Patient (n) | Outcomes (mo) | Jadad score |
|---|---|---|---|---|
| Kang (2012) | Retrospective Observational | 236 (SCD = 190, LCD = 46) | Total BMD; Calcium; ionized Calcium; i-PTH; ALP (24 month) | 1 |
| Sanchez (2004) | Prospective study; randomized | 24 (SCD = 10, LCD = 14) | Calcium; ionized calcium; i-PTH; phosphate; ALP (12 month) | 3 |
| Weinreich (1996) | Prospective; randomized; controlled | 64 (SCD = 29, LCD = 35) | Ionized calcium; i-PTH; phosphate; ALP (24 month) | 3 |
| Johnson (1996) | RCT; DB | 22 (SCD = 11, LCD = 11) | Phosphate; magnesium; calcium; ionized calcium; i-PTH; BMD z-score (12 month) | 5 |
RCT = randomized controlled trial; DB = double blinded; SCD = standard calcium dialysate; LCD = low calcium dialysate; BMD = bone mineral density; i-PTH = intact parathyroid hormone; ALP = Alkaline Phosphatase.
Mineral metabolism and i-PTH level
To make data consistent across all studies, mineral concentration was normalized to mmol/l. Meta-analysis showed 12-24 months after PD, both total and ionized calcium serum levels were lower in LCD patients as compared with SCD patients (Total calcium: MD, 0.09; 95% CI, 0.05 0.13; P < 0.0001; Ionized calcium: MD, 0.04; 95% CI, 0.02 0.06; P < 0.0001) (Figures 2 and 3). No statistical difference was observed in phosphate level between SCD and LCD group (MD, -0.05; 95% CI, -0.13 0.02; P = 0.19) (Figure 4). No heterogeneity was detected across these studies and fixed effects model was applied for the meta-analysis (Total calcium: I2 = 0%, P = 0.85; Ionized calcium: I2 = 31%, P = 0.22; Phosphate: I2 = 17%, P = 0.31).
Figure 2.
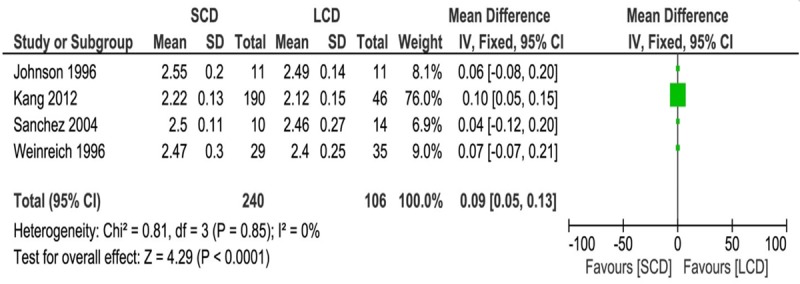
Forest plot of weighted mean difference with 95% confidence interval comparing total calcium level between SCD and LCD treatment in PD patients.
Figure 3.
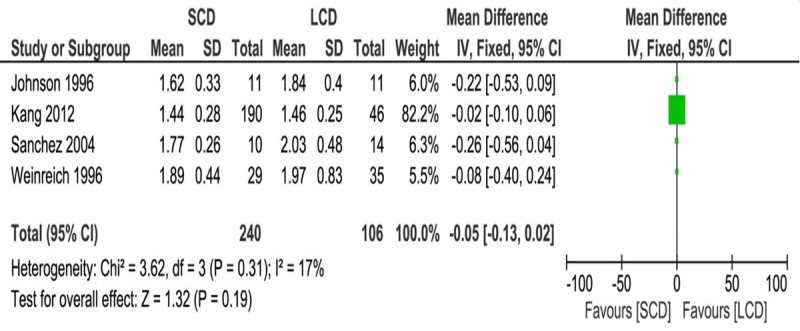
Forest plot of weighted mean difference with 95% confidence interval comparing ionized calcium level between SCD and LCD treatment in PD patients.
Figure 4.
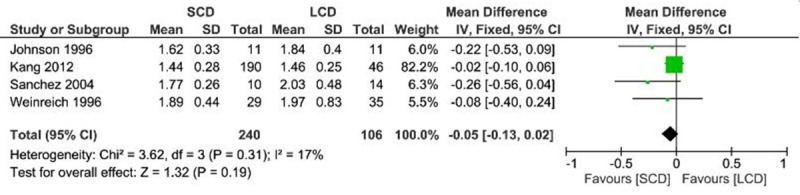
Forest plot of weighted mean difference with 95% confidence interval comparing phosphate level between SCD and LCD treatment in PD patients.
Serum I-PTH level and bone metabolism
Results of long term change of serum i-PTH level and bone metabolism from four studies could not be pooled together for meta-analysis and were summarized in Table 2. I-PTH level at 1-2 year after PD was significantly increased in LCD patients in three studies [6-8]. Johnson et al. observed increase of i-PTH at the 3-month and 6-month intervals in LCD patients [9]. However, No significant differences were detected between the two groups in the last six months of the study. For bone metabolism, Kang et al. showed decrease of BMD in LCD patients whereas Sanchez et al. observed a trend of rise in dynamic parameters (mineral apposition rate and bone formation rate) in LCD patients. Weinreich et al. and Johnson et al. did not show any difference in bone metabolism between LCD and SCD patients after 1-2 yr PD.
Table 2.
Comparison of long term i-PTH level and bone metabolism change between LCD and SCD patients after 1-2 yr PD
| study | Serum I-PTH | Bone Metabolism |
|---|---|---|
| Kang (2012) | SCD = 145.2 ± 155.4 (ng/l) | The adjusted 2 yr decrement of BMC, L-spine BMD, subtotal BMD and total BMD from base line was greater in the LCD group than in the SCD group |
| LCD = 213.2 ± 147.7 (ng/l) | ||
| At 2 yr; P = 0.008 | ||
| Sanchez (2004) | ΔPTH (SCD) = 21.3 ± 88.1 (pg/ml) | The rise in dynamic parameters (mineral apposition rate and bone formation rate) in LCD patients almost reached statistical significance (P = 0.07) |
| ΔPTH (LCD) = 174.4 ± 254.2 (pg/ml) at 1 yr; P < 0.05 | ||
| Weinreich (1996) | SCD = 7.5 (1-100) (pmol/l) | No difference was observed in radiological findings between LCD and SCD patients at 2 yr after PD |
| LCD = 17 (1-175) (pmol/l) at 2 yr; P < 0.05 | ||
| Johnson (1996) | At the 3-month and 6-month intervals, mean PTH levels were significantly higher in the LCD patients compared with SCD patients (P = 0.004 and 0.016 respectively). No significant differences were detected between the two groups in the last six months of the study. | Z-scores for total and regional BMDs did not differ between LCD and SCD group at 1 yr. |
SCD = standard calcium lysate; LCD = low calcium lysate; PTH = parathyroid hormone; BMC = bone mineral content; BMD = bone mineral density; PD = peritoneal dialysis.
Publication bias assessment
Begg’s funnel plot was used for the assessment of the publication bias of selected studies for meta-analysis. No publication bias was detected (Figure 5).
Figure 5.
Funnel plot for qualitative estimation of publication bias of the selected studies.
Discussion
LCD has become the first choice for PD patients taking calcium-containing phosphate binder to avoid aluminum exposure and reduce the incidence of hypercalcemia [10,11]. Indeed, our meta-analysis showed that after 1-2 year PD, serum total and ionized calcium level were much lower in LCD patients than in SCD patients. No difference was observed in serum phosphate level between LCD and SCD patients, indicating LCD in replacement of SCD is safe. In accordance with previous studies, serum PTH level increased in LCD patients after PD [11,12]. Interestingly, no correlation was observed between PTH level and bone metabolism in our study. High PTH level was accompanied by reduced BMD as reported in Kang et al.’s study but increased bone formation rate in Sanchez et al.’s study whereas no difference in bone metabolism was observed between LCD and SCD patients in other two studies.
PTH is a calciotropic hormone which usually enhances calcium reabsorption and calcium release. However, its action on skeleton is much more complex. It can stimulate both bone formation and reabsorption with net effect depending on the pattern of its administration [14-16]. Intermittent exposure to PTH leads to a net increase in bone formation, whereas its continuous administration results in bone loss. These effects may explain the different bone metabolism changes in response to increase of PTH in CLD patients. Despite the statistical difference in the change of bone metabolism existed in some of selected studies, none of these changes had reached clinical significance. The difference in the decline in total BMD between the LCD and SCD patients was only 3% per year in Kang et al.’s study. The increase of bone formation rate in LCD group in Sanchez et al.’s study did not reach statistical significance (P = 0.07).
The major limitation of our meta-analysis was lack of large size and randomized studies. Most of the patients included in the current analysis were from Kang et al., which was a prospective cohort study. Any patient selection bias or inaccurate endpoint results within this specific study would have significant impact on the accuracy of final meta-analysis result. Therefore, caution should be taken when considering long term efficacy and safety of LCD on PD patients based on our analysis results.
Conclusion
Long term (1-2 year) use of low calcium dialysate with 1.25 mmol/l calcium concentration in peritoneal dialysis patients results in decrease of serum total and ionized calcium level and has no effect on phosphate level. No clinical significance in the change of bone metabolism was observed between LCD and SCD patients despite the increase of serum parathyroid hormone in LCD group.
Acknowledgements
This work was supported by the National Key Technologies R&D Program during the Twelfth Five-year Plan Period (2011BAI10B08, 2014BAI11B16) and the Foundation for National Clinical Research Center (2015BAI12B06, 2013BAI09B05).
Disclosure of conflict of interest
None.
References
- 1.KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. Kidney Int Suppl. 2009;113:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 2.Cupisti A, Gallieni M, Rizzo MA, Caria S, Meola M, Bolasco P. Phosphate control in dialysis. Int J Nephrol Renovasc Dis. 2013;6:193–205. doi: 10.2147/IJNRD.S35632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinreich T, Passlick-Deetjen J, Ritz E. Low dialysate calcium in continuous ambulatory peritoneal dialysis: a randomized controlled multicenter trial. The Peritoneal Dialysis Multicenter Study Group. Am J Kidney Dis. 1995;25:452–60. doi: 10.1016/0272-6386(95)90108-6. [DOI] [PubMed] [Google Scholar]
- 4.Rotellar C, Kinsel V, Goggins M, Tarman G, Stull M, Mazzoni MJ, Mosher WF, Rakowski TA, Winchester JF. Does low-calcium dialysate accelerate secondary hyperparathyroidism in continuous ambulatory peritoneal dialysis patients? Perit Dial Int. 1993;13(Suppl 2):S471–2. [PubMed] [Google Scholar]
- 5.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 6.Kang SH, Cho KH, Park JW, Yoon KW, Do JY. Low-calcium dialysate as a risk factor for decline in bone mineral density in peritoneal dialysis patients. Scand J Urol Nephrol. 2012;46:454–60. doi: 10.3109/00365599.2012.700643. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez C, López-Barea F, Sánchez-Cabezudo J, Bajo A, Mate A, Martínez E, Selgas R Multicentre Study Group Collaborators. Low vs standard calcium dialysate in peritoneal dialysis: differences in treatment, biochemistry and bone histomorphometry. A randomized multicenter study. Nephrol Dial Transplant. 2004;19:1587–93. doi: 10.1093/ndt/gfh214. [DOI] [PubMed] [Google Scholar]
- 8.Weinreich T, Ritz E, Passlick-Deetjen J. Long-term dialysis with low-calcium solution (1.0 mmol/L) in CAPD: effects on bone mineral metabolism. Collaborators of the Multicenter Study Group. Perit Dial Int. 1996;16:260–8. [PubMed] [Google Scholar]
- 9.Johnson D, Rigby R, Mclntyre H, Brown A, Freeman J. A randomized trial comparing 1.25 mmol/1 calcium dialysate to 1.75 mmol/1 calcium dialysate in CAPD patients. Nephrol Dial Transplant. 1996;11:88–93. [PubMed] [Google Scholar]
- 10.Weinreich T, Passlick-Deetjen J, Ritz E. Low dialysate calcium in CAPD -a randomised controlled multicenter trial. Am J Kidney Dis. 1995;25:452–60. doi: 10.1016/0272-6386(95)90108-6. [DOI] [PubMed] [Google Scholar]
- 11.Hutchison A, Freemont AJ, Boulton HF, Gokal R. Low calcium dialysis fluid and oral calcium carbonate in CAPD. A method of controlling hyperphosphatemia whilst minimising aluminum exposure and hypercalcemia. Nephrol Dial Transplant. 1992;7:1219–25. doi: 10.1093/ndt/7.12.1219. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong A, Beer J, Noonan K, Cunningham J. Reduced calcium dialysate in CAPD patients: efficacy and limitations. Nephrol Dial Transpl. 1997;12:1223–1228. doi: 10.1093/ndt/12.6.1223. [DOI] [PubMed] [Google Scholar]
- 13.Argilés A, Mourad G. How do we have to use the calcium in the dialysate to optimize the management of secondary hyperparathyroidism? Nephrol Dial Transpl. 1998;13(Suppl 3):62–64. doi: 10.1093/ndt/13.suppl_3.62. [DOI] [PubMed] [Google Scholar]
- 14.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–46. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest. 2011;34:801–10. doi: 10.3275/7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esbrit P, Alcaraz MJ. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem Pharmacol. 2013;85:1417–23. doi: 10.1016/j.bcp.2013.03.002. [DOI] [PubMed] [Google Scholar]


