Abstract
We tested the hypothesis that large areas of small hard drusen (diameter <63 µm) and intermediate drusen (diameter 63–124 µm) are associated with the incidence of age-related macular degeneration (AMD). Eyes of 3344 older adults with at least two consecutive visits spaced five years apart over a 20-year period were included. A 6-level severity scale, including no drusen, four levels of increasing area (from minimal (<2596 µm²) to large (>9086 µm²)) of only small hard drusen, and intermediate drusen, was used. The five-year incidence of AMD was 3% in eyes at the start of the interval with no, minimal, small, and moderate areas of only small drusen and 5% and 25% for eyes with large area of only small drusen and intermediate drusen, respectively. Compared to eyes with a moderate area of small drusen, the odds ratio (OR) of developing AMD in eyes with a large area of only small drusen was 1.8 (p < 0.001). Compared to eyes with large area of only small drusen, eyes with intermediate drusen had an OR of 5.5 (p < 0.001) of developing AMD. Our results are consistent with our hypothesis that large areas of only small drusen are associated with the incidence of AMD.
Keywords: age-related macular degeneration, epidemiology, genetic risk, incidence, retinal drusen, risk factors, size and area
1. Introduction
Age-related macular degeneration (AMD) is a complex chronic disease affecting multiple layers of the retina. Histologically, its earliest subclinical stages have been defined by the presence of age-related thickening of Bruch membrane with calcifications and basal laminar deposits prior to the appearance of hard and soft drusen in the macula as detected by ophthalmoscopy [1]. Clinically, its earliest stages are often defined by the presence of large soft drusen (≥125 µm in diameter) [2,3]. Progression of AMD involves the appearance of increasing numbers of large soft distinct and indistinct drusen that may become confluent, as well as the development of pigmentary abnormalities (depigmentation and increased retinal pigment) of the retinal pigment epithelium (RPE) in the macula. Progression rates vary, with some eyes remaining in early stages of the disease over long periods of time while others progress more rapidly to the late stages of the disease, geographic atrophy and neovascular AMD. Reduction in choroidal perfusion and retinal tissue oxygenation levels, increased systemic inflammation, presence of atherosclerosis, and elastic tissue degeneration have been hypothesized to be involved in the pathogenesis of AMD [3,4]. Genes associated with the alternative complement system, lipid metabolism, atherosclerosis, and other pathways have been found to be associated with the development of late AMD [5,6].
Labeling an eye as having early AMD has been based on empirical evidence from epidemiological studies (e.g., the Beaver Dam Eye Study, the Rotterdam Study, the Blue Mountains Eye Study), clinical trials, such as the Age-Related Eye Disease Study (AREDS) and observations of clinical cohorts [2,3,7,8,9,10,11,12]. In those studies, the presence of large drusen (≥125 µm in diameter) in one or both eyes increased the risk of developing signs of late AMD [2,3,11]. In the AREDS, the presence of intermediate drusen (between 63 and 124 µm in diameter) in both eyes was also associated with a small but significant increase in risk of developing late AMD [2,11]. Presence of a few small hard drusen is common and is not thought to be an early stage of AMD [13,14]. Eyes with a larger area of only small hard drusen (>9086 µm2) compared with eyes with a smaller drusen area (<2596 µm²) involving the macula have been found to be associated with increased age-adjusted 15-year cumulative incidence of soft indistinct drusen (16.3% vs. 4.7%) and pigmentary abnormalities (10.6% vs. 2.7%) [7]. These findings suggested that a larger area of small hard drusen in the absence of larger soft drusen or other retinal AMD lesions in an eye might be an even earlier clinical stage of AMD than that which is currently defined by the presence of larger drusen.
We hypothesized that larger areas of only small hard drusen and the presence of intermediate drusen were related to the incidence of AMD. We also hypothesized that age, sex, and two specific single-nucleotide polymorphisms (SNPs), one in the complement factor H region (CFH Y402H rs1061170) on chromosome 1 and the other in the age-related maculopathy susceptibility 2 region (ARMS2 A69S rs10490924) on chromosome 10, were related to the incidence of increasing areas of small hard drusen and to intermediate drusen. Longitudinal data over a 20-year period from the Beaver Dam Eye Study were used in the analyses.
2. Experimental Section
2.1. Study Population
Identification and descriptions of the population in the Beaver Dam Eye Study have appeared in previous reports [15,16,17,18,19,20]. In brief, a private census identified 5924 persons living in the city and township of Beaver Dam, Wisconsin aged 43–86 years, of which 4926 participated in the baseline examination in 1988–1990 and in up to four follow-up examinations in 1993–1995, 1998–2000, 2003–2005 and 2008–2010. Over 99% of the population was white. All subjects gave their informed consent for inclusion before they participated in the study. The investigations were carried out following the rules of the Declaration of Helsinki, and the research protocol was approved by the institutional review board at the University of Wisconsin-Madison. Comparisons between participants and nonparticipants at each examination have appeared elsewhere [15,16,17,18,19,20]. In general, those who participated in a follow-up examination over the 20-year period of the study were more likely to be younger than nonparticipants who were alive or those who died before follow-up and, with adjustment for age, were less likely to have early AMD as defined as having severity level 20 or greater on the 5-step Three Continent Consortium AMD severity scale in at least 1 eye [9].
2.2. Procedures
The baseline and follow-up examinations used similar procedures, which have been described in detail elsewhere [2,4,13,21,22,23,24,25,26,27]. Stereoscopic 30° color film fundus photographs centered on the disc (Diabetic Retinopathy Study standard field 1) and macula (Diabetic Retinopathy Study standard field 2) and a nonstereoscopic color fundus photograph temporal to but including the fovea of each eye were obtained. Details of the grading procedure have been described elsewhere [2,4,13,21,22,23,24,25,26,27].
2.3. Genetic Measurements
The genetic measurements have been described in detail elsewhere [28]. In brief, samples of DNA were extracted from buffy coat specimens collected at the baseline examination. The two SNPs most commonly associated with AMD, Y402H in CFH (rs1061170) and A69S in ARMS2 (rs10490924), were used in this study. For CFH, T was considered the wild allele and C the risk allele; for ARMS2, G was considered the wild allele and T the risk allele.
2.4. Definitions
Age was documented at each participant visit and was categorized as <60 years, 60–69 years, or ≥70 years. Body mass index was calculated by dividing a participant’s weight in kilograms by his or her height in meters squared. Current heavy drinking was defined as the consumption of 4 or more servings of alcoholic beverages daily at the time of the examination; a serving was defined as 12 fluid ounces of beer, 4 fluid ounces of wine, or 1.5 fluid ounces of liquor. Participants were considered physically active if they engaged in physical activity long enough to work up a sweat at least 3 times per week. Hypertension was defined as blood pressure ≥140/90 mmHg or use of anti-hypertensive medication. Aspirin, multivitamin, and anti-hypertensive medication use were determined from self-report.
The severity status of drusen in an eye was defined as follows.
Level 1 (No drusen): No signs of any drusen or of early or late AMD.
Level 2 (Minimal area of small hard distinct drusen): Presence of small hard distinct drusen <63 µm in diameter as the largest size drusen involving a circular area of the macula of up to 2596 µm² and no other signs of AMD.
Level 3 (Small area of small hard distinct drusen): Presence of small hard distinct drusen <63 µm in diameter as the largest size drusen involving a circular area of the macula between 2597 µm² and 5192 µm² and no other signs of AMD.
Level 4 (Moderate area of small hard distinct drusen): Presence of small hard distinct drusen <63 µm in diameter as the largest size drusen involving a circular area of the macula between 5193 µm² and 9086 µm² and no other signs of AMD.
Level 5 (Large area of small hard distinct drusen): Presence of small hard distinct drusen <63 µm in diameter as the largest size drusen involving a circular area of the macula greater than 9086 µm² and no other signs of AMD.
Level 6 (Intermediate drusen): Presence of one or more drusen 63 to 124 µm in diameter as the largest size drusen and no other signs of AMD.
Early AMD was defined as the presence of small to intermediate drusen, regardless of area of involvement, with any pigmentary abnormalities (defined as depigmentation and increased retinal pigment) or the presence of large drusen (≥125 µm in diameter). Late AMD was defined by the presence of geographic atrophy and signs of exudative AMD, such as serous detachment of the RPE and/or sensory retina.
2.5. Statistical Analysis
We examined the association of the drusen severity level in an eye and other risk factors measured at one examination with drusen severity level in that eye at the next examination, 5 years later. Pairs of visits were accumulated during the 20 years of the study and risk factors were updated for each 5-year period. Multiple contributions from an individual were accounted for by using generalized estimating equations (GEE).
An eye was considered at risk for incidence of a specific level on the drusen severity scale if drusen were gradable at 2 consecutive visits and had a less severe level of drusen at the earlier examination. The outcome of interest was the development of drusen at or above a given severity level (e.g., intermediate drusen or any AMD). For example, development of intermediate drusen (Level 6) was evaluated for all eyes with Level 1 through Level 5 at the start of an interval. If the severity level was Level 6 or any AMD at the follow-up visit, the eye was considered to have developed intermediate drusen.
Analyses of specific risk factors (age, sex, genotype) were stratified by the initial drusen severity level. Models were adjusted for age and sex. SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
3. Results
3.1. Participant Characteristics
There were 3344 individuals who contributed AMD data for at least one pair of consecutive examinations: 3198 with baseline and five-year follow-up data, 2367 participants with five-year and 10-year follow-up data, 1865 participants with 10-year and 15-year follow-up data, and 1468 participants with 15-year and 20-year follow-up data. For each interval, 75%–80% of individuals had data available for both eyes, resulting in 15821 total person-eye-visit intervals. Table 1 shows the characteristics of this group. The percentages of individuals at the start of a five-year follow-up interval with Level 1, Level 2, Level 3, Level 4, Level 5, and Level 6 were 14%, 26%, 16%, 15%, 14% and 16%, respectively (Table 1). The genotype distributions for CFH Y402H were 41%, 46% and 13% for T/T, T/C, and C/C, respectively; the genotype distributions for ARMS2 A69S were 61%, 35%, and 4% for G/G, G/T, and T/T, respectively.
Table 1.
Characteristics of individuals included in analyses averaged over all examination phases in the Beaver Dam Eye Study, 1988–1990 to 2008–2010. ARMS2, age-related maculopathy susceptibility 2 rs10490924; CFH, complement factor H rs1061170; SD, standard deviation.
| Characteristic | Person-Eye-Visits (N) | Mean (SD) or % | |
|---|---|---|---|
| Age, years | 15821 | 62.3 (9.5) | |
| Sex | Female | 9016 | 57.0 |
| Male | 6805 | 43.0 | |
| Beginning drusen severity level | Level 1 | 2206 | 13.9 |
| Level 2 | 4055 | 25.6 | |
| Level 3 | 2515 | 15.9 | |
| Level 4 | 2370 | 15.0 | |
| Level 5 | 2210 | 14.0 | |
| Level 6 | 2465 | 15.6 | |
| CFH genotype | T/T | 5942 | 40.6 |
| T/C | 6771 | 46.3 | |
| C/C | 1919 | 13.1 | |
| ARMS2 genotype | G/G | 9172 | 60.9 |
| G/T | 5323 | 35.3 | |
| T/T | 574 | 3.8 | |
| Hypertension present | No | 7911 | 50.4 |
| Yes | 7776 | 49.6 | |
| Body mass index, kg/m² | 15625 | 29.6 (5.6) | |
| Smoking history | Never | 7312 | 46.2 |
| Past | 6189 | 39.1 | |
| Current | 2312 | 14.6 | |
| Heavy drinking history | Never | 13377 | 84.6 |
| Past | 2154 | 13.6 | |
| Current | 276 | 1.7 | |
| Physical activity level | Active | 4843 | 30.6 |
| Sedentary | 10969 | 69.4 | |
| Using multivitamins | No | 7720 | 48.8 |
| Yes | 8101 | 51.2 | |
| Currently using aspirin | No | 10946 | 69.3 |
| Yes | 4854 | 30.7 |
3.2. Incidence of Varying Areas of Small Hard Drusen, Intermediate Drusen, and Any AMD
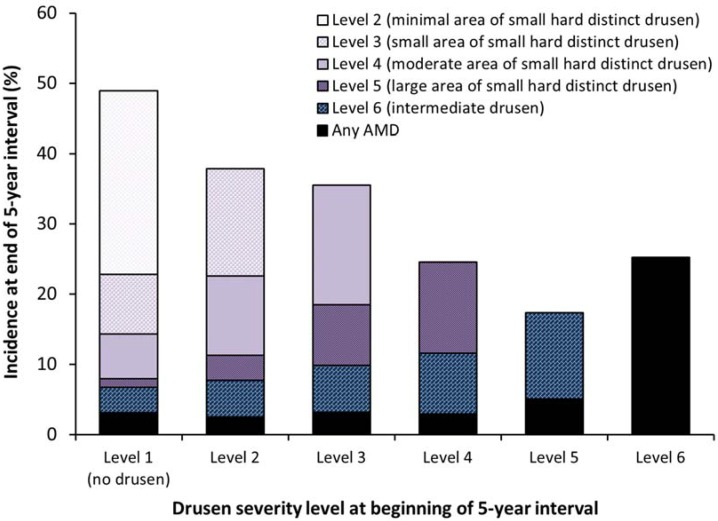
Figure 1 shows the distribution of drusen severity levels at the follow-up examination for each level at the start of the interval and Table 2 shows the results of incidence analyses for each drusen severity level. As severity level at the beginning of an interval increased, so did the development of the more severe drusen levels (Figure 1). For example, 8% of eyes with Level 1 (n = 2206) at the start of a five-year follow-up interval developed Level 5 or worse compared with 25% of eyes starting at Level 4 (n = 2370). 49% of eyes with Level 1 (n = 2206) developed Level 2 or worse by the end of a five-year period, and 36% of eyes with Level 3 (n = 2515) developed Level 4 or worse.
Figure 1.
The relationship of no drusen, 4 areas of increasing involvement of the macula by small hard drusen and intermediate drusen to the 5-year incidence of more severe levels of drusen and any AMD in the Beaver Dam Eye Study, 1988–1990 to 2008–2010.
Table 2.
Incidence of specific level of drusen area/severity by initial severity level. N is the number of person-visit-eyes at risk. The p value is the Wald test for any differences between levels. AMD, age-related macular degeneration; CI, confidence interval; OR, odds ratio.
| Incidence of | Beginning Level | N at Risk | % Incident | Comparison Level | OR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Level 2 or worse | Level 1 | 2206 | 48.9 | |||
| Level 3 or worse | Level 1 | 2206 | 22.8 | <0.001 | ||
| Level 2 | 4055 | 37.8 | Level 1 | 2.0 (1.8, 2.3) | ||
| Level 4 or worse | Level 1 | 2206 | 14.3 | <0.001 | ||
| Level 2 | 4055 | 22.5 | Level 1 | 1.7 (1.5, 1.9) | ||
| Level 3 | 2515 | 35.5 | Level 2 | 1.9 (1.7, 2.1) | ||
| Per level | 1.8 (1.7, 1.9) | <0.001 | ||||
| Level 5 or worse | Level 1 | 2206 | 7.9 | <0.001 | ||
| Level 2 | 4055 | 11.3 | Level 1 | 1.4 (1.2, 1.7) | ||
| Level 3 | 2515 | 18.5 | Level 2 | 1.7 (1.5, 2.0) | ||
| Level 4 | 2370 | 24.5 | Level 3 | 1.4 (1.2, 1.6) | ||
| Per level | 1.5 (1.5, 1.6) | <0.001 | ||||
| Level 6 or worse | Level 1 | 2206 | 6.8 | <0.001 | ||
| Level 2 | 4055 | 7.7 | Level 1 | 1.2 (1.0, 1.4) | ||
| Level 3 | 2515 | 9.8 | Level 2 | 1.3 (1.1, 1.5) | ||
| Level 4 | 2370 | 11.6 | Level 3 | 1.2 (1.0, 1.4) | ||
| Level 5 | 2210 | 17.3 | Level 4 | 1.5 (1.3, 1.8) | ||
| Per level | 1.3 (1.2, 1.4) | <0.001 | ||||
| Any AMD | Level 1 | 2206 | 3.1 | <0.001 | ||
| Level 2 | 4055 | 2.5 | Level 1 | 0.8 (0.6, 1.1) | ||
| Level 3 | 2515 | 3.1 | Level 2 | 1.2 (0.9, 1.6) | ||
| Level 4 | 2370 | 2.9 | Level 3 | 0.9 (0.7, 1.3) | ||
| Level 5 | 2210 | 5.1 | Level 4 | 1.8 (1.3, 2.4) | ||
| Level 6 | 2465 | 25.2 | Level 5 | 5.5 (4.5, 6.8) | ||
| Per level | 1.8 (1.7, 1.9) | <0.001 |
Five-year incidence of Level 6 or worse was 17%, 12%, 10%, 8%, and 7% for individuals with Level 5, Level 4, Level 3, Level 2, and Level 1 at the start of the interval, respectively. Incidence of any AMD was higher in eyes with Level 6 at the start of the interval compared to eyes with Level 5 (25% vs. 5%; odds ratio [OR] = 5.5) and was also higher in eyes with Level 5 vs. Level 4 at the beginning of a five-year period (OR = 1.8).
3.3. Relationships of Age, Sex and Two AMD Candidate Genes to the Five-Year Incidences of Small Hard Drusen, Intermediate Drusen and AMD
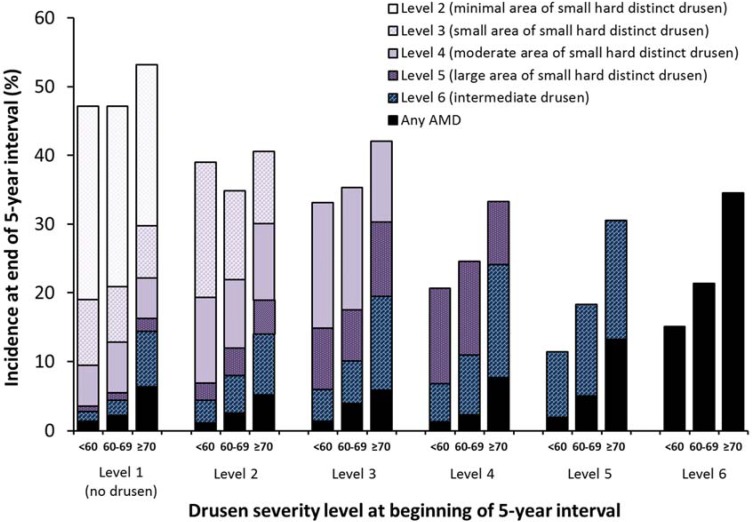
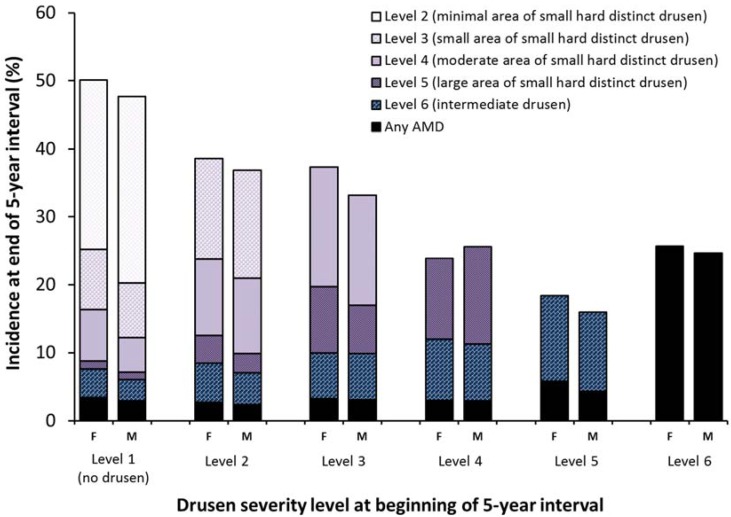
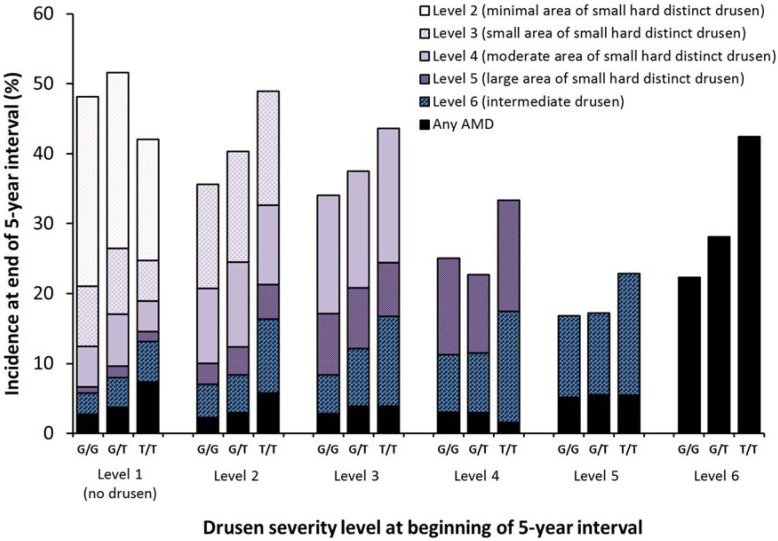
Figure 2, Figure 3, Figure 4, Figure 5 and Supplementary Tables S1–S6 show the relationships between drusen severity level and AMD status at follow-up by age, sex and genetic information, stratified by initial drusen level. Figure 2 shows the distribution of the follow-up drusen severity level and AMD status by age group. Odds ratios for these relationships are presented in Supplementary Tables S1–S6, with each possible outcome in a separate table. Older age was associated with higher five-year incidence of most outcomes and the associations were stronger in eyes with larger areas of small hard drusen and more severe lesions. Sex was not associated with the development of any drusen outcome (Figure 3).
Figure 2.
Five-year incidence of increasing area of small hard drusen, intermediate drusen, and any AMD by age group (<60, 60–69, and ≥70 years) and severity level at the beginning of the interval.
Figure 3.
Five-year incidence of increasing area of small hard drusen, intermediate drusen, and any AMD by sex and severity level at the beginning of the interval. F = female; M = male.
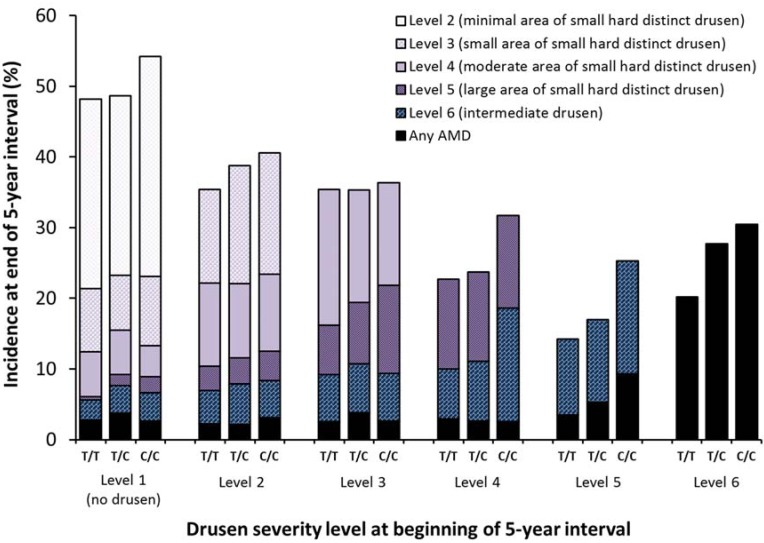
Figure 4.
Five-year incidence of increasing area of small hard drusen, intermediate drusen, and any AMD by CFH Y402H (rs1061170) genotype and severity level at the beginning of the interval.
Figure 5.
Five-year incidence of increasing area of small hard drusen, intermediate drusen, and any AMD by ARMS2 A69S (rs10490924) genotype and severity level at the beginning of the interval.
Figure 4 and Figure 5 and Supplementary Tables S1–S6 show the relationships of the CFH and ARMS2 candidate genes stratified by drusen severity levels at the beginning of an interval to the five-year incidences for each drusen severity level and AMD outcome. While few individuals had two risk alleles, especially for ARMS2, the increasing incidence was still apparent and statistically significant for some relationships. For CFH Y402H genotype (Figure 3), the strongest associations for incidence of Level 6 (Supplementary Table S5) and incidence of any AMD (Supplementary Table S6) occurred when the initial severity was at least Level 4. Incidence of Level 6 was about 9%–10% regardless of the number of risk alleles for CFH Y402H (p = 0.35 for any differences after adjusting for age and sex) when initial drusen severity was Level 3 but ranged from 14% to 25% for 0 vs. 2 risk alleles when the initial severity was Level 5 (OR = 1.4 per risk allele, p < 0.001 after adjusting for age and sex). Where an association with CFH was present, the association was stronger in individuals with 2 risk alleles compared to individuals with only one risk allele.
For ARMS2 A69S genotype (Figure 4), most of the strongest associations were for smaller areas of drusen at the start of an interval. Having two risk alleles was more strongly associated with incidence than having one risk allele present. For example, incidence of Level 6 was higher when there were two risk alleles compared to one or 0, but was only statistically significant when the initial level of drusen severity was Level 1, 2, or 3. Among individuals with Level 1, 13% of those with the T/T genotype (2 risk alleles), 8% of the G/T (1 risk allele) and 6% of those with G/G (0 risk alleles) developed Level 6 over the next five years (OR = 1.7 per risk allele, p = 0.002 after adjusting for age and sex). Among persons with Level 5, 17%, 17% and 23% of those with 0, 1 and 2 risk alleles, respectively, developed Level 6 (OR = 1.2 per risk allele, p = 0.16 after adjusting for age and sex).
4. Discussion
There is a general consensus that large soft drusen and pigmentary abnormalities define an early AMD phenotype and that neovascular AMD and pure geographic atrophy define the late advanced AMD phenotype. However, there is less agreement regarding whether a large area of small hard distinct drusen defines an even earlier AMD phenotype. In the Beaver Dam Eye Study, we found that the incidence of increasingly larger areas of small hard drusen (more severe level) is dependent upon the prior less severe level (smaller area) of small hard drusen. Even an eye with a minimal area of small hard drusen (Level 2) has approximately 50% greater odds of progressing by one step than an eye with no drusen (Level 1). While there is some variability, a stepwise increase in severity seems reasonable to assume. Furthermore, the data are consistent with the notion that there is an increasing likelihood of progression to intermediate drusen or any AMD with a larger area of small hard drusen. We had previously reported that a large area >9086 µm²) of small hard distinct drusen compared to a small area (≤2596 µm²) of involvement of these drusen in the absence of any other signs of AMD was associated with increased 15-year cumulative incidence of early AMD (18% vs. 5%) and late AMD (1.5% vs. 0.4%) [7]. This was consistent with earlier observations from clinical studies suggesting that eyes with large numbers of small hard drusen were at greater risk of developing AMD [29,30,31].
The process by which large soft drusen develop and their relation to small hard drusen is poorly understood [29,31,32,33]. Large soft drusen have been thought to result either from the aggregation of small hard drusen or in areas where small hard drusen appear to be absent. Hard and soft drusen have differing molecular components. Sarks et al. and others [14,34,35] have described the focal aggregation of small hard drusen into mounds of membranous debris, which accumulate as a widespread shallow layer between the basement membrane of the pigment epithelium and Bruch membrane. Small subclinical sized drusen (<30 µm in diameter) not usually visible on ophthalmoscopy have been found to become confluent and form soft drusen [31]. Areas surrounding individual large soft drusen have been shown to contain several small merging drusen and thick basal laminar drusen. Further understanding of the biochemical, physiological, and anatomic changes that take place as large soft drusen evolve is needed.
We wanted to examine whether age and different high risk AMD candidate genes might affect different stages of drusen development in addition to the natural history of drusen development. In the Beaver Dam Eye Study, genetic associations from CFH Y402H and ARMS2 A69S appear to affect different stages of drusen development. Some of these differences are related to underpowered tests with ARMS2 but may also represent differences in the genetic influences on disease.
In the present report, besides the differing associations of the SNPs of two genes associated with AMD (CFH Y402H rs1061190 and ARMS2 A69S rs10490924), we found relationships of age but not sex to the incidence of increasing areas of macular involvement with small hard drusen, intermediate drusen, and AMD. It is difficult to compare our findings with those of other population-based studies that have examined the incidence and progression of small hard distinct drusen due to differences in the criteria defining the drusen endpoint among the studies. In the Inter99 Eye Study [36], there was no relationship between the presence of 20 or more small hard macular drusen with gene SNPs CFH Y402H, LOC387715 A69S, HTRA1 rs11200638, CFB R32Q, and CFB L9H while drusen >63 µm in diameter were related to CFH Y402H. The Münster Aging and Retina Study (MARS) [37] examined drusen features such as number, confluence, type, size, and area occupied by macular drusen in 406 patients with early AMD and 170 healthy controls with a follow-up examination after 2.6 years. In that study, over a 2½ year period, CFH Y402H but not ARMS2 A69S was associated with the development of 20 or more small hard drusen in eyes described as healthy at baseline. In another study, investigators developing a new clinical classification system for AMD concluded that small drusen (<63 µm) should be considered to be a result of normal aging changes and that there was no clinically relevant increased risk of late AMD developing in these eyes [30]. In that study, persons with intermediate drusen (63 to 124 µm in diameter) were considered to have early AMD. These findings from the Beaver Dam Eye Study and other studies suggest that intermediate drusen may be one of the earliest stages of AMD clinically identifiable by grading of fundus photographs. Others have shown an increased risk of developing AMD when intermediate drusen are present [30,36,37].
Our study has many strengths, including repeated examinations during a 20-year period that used detailed standardized procedures for obtaining stereoscopic color fundus photographs of the macula and an objective system for grading those photographs for AMD phenotypes. However, the study also had a number of limitations. First, the Beaver Dam Eye Study cohort is racially/ethnically homogeneous (over 99% white), which limits our inferences regarding these findings in nonwhites. Second, we used SNPs from only two AMD candidate genes; it is possible that inclusion of all the identified possible loci may have affected our findings. Non-AMD related processes may cause the development of the first few small hard macular drusen. There may also be more misclassification in eyes with no or only small drusen because it is more difficult to detect some small hard drusen, leading to greater variability in grading their presence than in eyes with larger drusen or more severe late signs of AMD and resulting in detection bias due to size. Mortality may also have limited the interpretation of associations because of selective survival.
5. Conclusions
This report provides long-term population-based observations regarding the relationships of age, sex, and presence of risk alleles for two AMD candidate genes, CFH Y402H rs1061170 and ARMS2 A69S rs10490924, to the incidence of small hard drusen, as well as their role in the natural history of AMD in its earliest clinical stages using grading of stereoscopic 30° color fundus film photography. The findings from the Beaver Dam Eye Study and others provide some evidence that intermediate drusen and large areas of small hard drusen may be early clinically detectable stages in the trajectory of the disease and not just risk indicators. Intervals of observation of the cohort longer than five years may be needed to provide enough time to evaluate the natural history of no or a few small drusen progressing to AMD. This will be evaluated by multistate modeling in future analyses. Our findings also suggest the genetic risks from CFH and ARMS2 may influence different stages of the disease in different ways. Inclusion of newer technological assessment tools such as spectral domain optical coherence tomography and autofluorescence may provide more sensitive and reliable approaches to detect and quantitate even earlier stages of AMD [33]. This may prove useful in the study of pathogenetic risk factors only associated with these earlier stages of AMD. However, risks and benefits of defining an individual as having early AMD when newer classifications are used in clinical practice will need to be examined further.
Acknowledgments
This study was supported by National Institutes of Health grant EY06594 (Barbara E. K. Klein and Ronald Klein) and, in part, by Research to Prevent Blindness, New York, NY. No funds were received for covering the costs to publish in open access. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish results. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
The authors acknowledge Heidi M. G. Christian, and Erin L. Syth, for technical assistance and editing of the manuscript.
Supplementary Files
Author Contributions
Ronald Klein conceived and designed the analysis. Ronald Klein, Barbara E. K. Klein, Theru A. Sivakumaran and Sudha K. Iyengar performed data collection. Chelsea E. Myers and Kristine E. Lee analyzed the data. Ronald Gangnon contributed analysis tools and statistical advice. Ronald Klein wrote the initial manuscript draft. All authors contributed to critical review and revision of the manuscript draft.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Van der Schaft T.L., Mooy C.M., de Bruijn W.C., Oron F.G., Mulder P.G., de Jong P.T. Histologic features of the early stages of age-related macular degeneration. A statistical analysis. Ophthalmology. 1992;99:278–286. doi: 10.1016/s0161-6420(92)31982-7. [DOI] [PubMed] [Google Scholar]
- 2.Ferris F.L., Davis M.D., Clemons T.E., Lee L.Y., Chew E.Y., Lindblad A.S., Milton R.C., Bressler S.B., Klein R. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch. Ophthalmol. 2005;123:1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R., Klein B.E., Jensen S.C., Meuer S.M. The five-year incidence and progression of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/S0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 4.Seddon J.M. Genetic and environmental underpinnings to age-related ocular diseases. Investig. Ophthalmol. Vis. Sci. 2013;54:ORSF28–ORSF30. doi: 10.1167/iovs.13-13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buitendijk G.H., Rochtchina E., Myers C., van Duijn C.M., Lee K.E., Klein B.E., Meuer S.M., de Jong P.T., Holliday E.G., Tan A.G., et al. Prediction of age-related macular degeneration in the general population: The Three Continent AMD Consortium. Ophthalmology. 2013;120:2644–2655. doi: 10.1016/j.ophtha.2013.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein M.L., Ferris F.L., III, Francis P.J., Lindblad A.S., Chew E.Y., Hamon S.C., Ott J. Progression of geographic atrophy and genotype in age-related macular degeneration. Ophthalmology. 2010;117:1554–1559. doi: 10.1016/j.ophtha.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein R., Klein B.E., Knudtson M.D., Meuer S.M., Swift M., Gangnon R.E. Fifteen-year cumulative incidence of age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell P., Wang J.J., Foran S., Smith W. Five-year incidence of age-related maculopathy lesions: The Blue Mountains Eye Study. Ophthalmology. 2002;109:1092–1097. doi: 10.1016/S0161-6420(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 9.Klein R., Meuer S.M., Myers C.E., Buitendijk G.H., Rochtchina E., Choudhury F., de Jong P.T., McKean-Cowdin R., Iyengar S.K., Gao X., et al. Harmonizing the classification of age-related macular degeneration in the Three Continent AMD consortium. Ophthalmic Epidemiol. 2014;21:14–23. doi: 10.3109/09286586.2013.867512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaver C.C., Assink J.J., van L.R., Wolfs R.C., Vingerling J.R., Stijnen T., Hofman A., de Jong P.T. Incidence and progression rates of age-related maculopathy: The Rotterdam Study. Investig. Ophthalmol. Vis. Sci. 2001;42:2237–2241. [PubMed] [Google Scholar]
- 11.Davis M.D., Gangnon R.E., Lee L.Y., Hubbard L.D., Klein B.E., Klein R., Ferris F.L., Bressler S.B., Milton R.C. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch. Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seddon J.M., Sharma S., Adelman R.A. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006;113:260–266. doi: 10.1016/j.ophtha.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Klein R., Klein B.E., Linton K.L. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/S0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 14.Gass J.D. Drusen and disciform macular detachment and degeneration. Arch. Ophthalmol. 1973;90:206–217. doi: 10.1001/archopht.1973.01000050208006. [DOI] [PubMed] [Google Scholar]
- 15.Klein R., Klein B.E., Lee K.E., Cruickshanks K.J., Chappell R.J. Changes in visual acuity in a population over a 10-year period: The Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–1766. doi: 10.1016/S0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 16.Klein R., Klein B.E., Lee K.E., Cruickshanks K.J., Gangnon R.E. Changes in visual acuity in a population over a 15-year period: The Beaver Dam Eye Study. Am. J. Ophthalmol. 2006;142:539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Klein R., Klein B.E., Lee K.E. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–1178. doi: 10.1016/S0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 18.Klein R., Lee K.E., Gangnon R.E., Klein B.E. Incidence of visual impairment over a 20-year period: The Beaver Dam Eye Study. Ophthalmology. 2013;120:1210–1219. doi: 10.1016/j.ophtha.2012.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein R., Klein B.E., Linton K.L., de Mets D.L. The Beaver Dam Eye Study: Visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/S0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 20.Linton K.L., Klein B.E., Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am. J. Epidemiol. 1991;134:1438–1446. doi: 10.1093/oxfordjournals.aje.a116049. [DOI] [PubMed] [Google Scholar]
- 21.Klein R., Klein B.E., Tomany S.C., Meuer S.M., Huang G.H. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology. 2002;109:1767–1779. doi: 10.1016/S0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 22.Klein R., Klein B.E. The Beaver Dam Eye Study II. Manual of Operations. National Technical Information Sevice; Springfield, Virginia, USA: 1995. Accession No. PB95-273827. [Google Scholar]
- 23.Klein R., Klein B.E. The Beaver Dam Eye Study III. Manual of Operations. National Technical Information Service; Springfield, Virginia, USA: 1999. Accession No. PB99-137861. [Google Scholar]
- 24.Klein B.E., Klein R. The Beaver Dam Eye Study V. Manual of Operations. National Technical Information Sevice; Springfield, Virginia, USA: 2010. Accession No. PB2010-114194. [Google Scholar]
- 25.Klein R., Klein B.E. The Beaver Dam Eye Study. Manual of Operations, Revised. National Technical Information Sevice; Springfield, Virginia, USA: 1991. Accession No. PB91-149823. [Google Scholar]
- 26.Klein R., Davis M.D., Magli Y.L., Segal P., Klein B.E., Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/S0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 27.Klein R., Davis M.D., Magli Y.L., Segal P., Klein B.E., Hubbard L. National Technical Information Sevice; Springfield, Virginia, USA: 1991. The Wisconsin Age-Related Maculopathy Grading System. Accession No. PB91-184267. [DOI] [PubMed] [Google Scholar]
- 28.Klein R., Myers C.E., Meuer S.M., Gangnon R.E., Sivakumaran T.A., Iyengar S.K., Lee K.E., Klein B.E. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: The Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131:383–392. doi: 10.1001/jamaophthalmol.2013.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardeljan D., Chan C.C. Aging is not a disease: Distinguishing age-related macular degeneration from aging. Prog. Retin. Eye Res. 2013;37:68–89. doi: 10.1016/j.preteyeres.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferris F.L., III, Wilkinson C.P., Bird A., Chakravarthy U., Chew E., Csaky K., Sadda S.R. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudolf M., Seckerdieck K., Grisanti S., Curcio C.A. Internal structure consistent with remodelling in very small drusen, revealed by filipin histochemistry for esterified cholesterol. Br. J. Ophthalmol. 2014;98:698–702. doi: 10.1136/bjophthalmol-2013-304226. [DOI] [PubMed] [Google Scholar]
- 32.Rudolf M., Clark M.E., Chimento M.F., Li C.M., Medeiros N.E., Curcio C.A. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Investig. Ophthalmol. Vis. Sci. 2008;49:1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yehoshua Z., Wang F., Rosenfeld P.J., Penha F.M., Feuer W.J., Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118:2434–2441. doi: 10.1016/j.ophtha.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gragoudas E.S., Chandra S.R., Friedman E., Klein M.L., Van B.M. Disciform degeneration of the macula. II. Pathogenesis. Arch. Ophthalmol. 1976;94:755–757. doi: 10.1001/archopht.1976.03910030365003. [DOI] [PubMed] [Google Scholar]
- 35.Sarks S.H., Van D.D., Maxwell L., Killingsworth M. Softening of drusen and subretinal neovascularization. Trans. Ophthalmol. Soc. UK. 1980;100:414–422. [PubMed] [Google Scholar]
- 36.Munch I.C., Ek J., Kessel L., Sander B., Almind G.J., Brondum-Nielsen K., Linneberg A., Larsen M. Small, hard macular drusen and peripheral drusen: Associations with AMD genotypes in the Inter99 Eye Study. Investig. Ophthalmol. Vis. Sci. 2010;51:2317–2321. doi: 10.1167/iovs.09-4482. [DOI] [PubMed] [Google Scholar]
- 37.Dietzel M., Pauleikhoff D., Arning A., Heimes B., Lommatzsch A., Stoll M., Hense H.W. The contribution of genetic factors to phenotype and progression of drusen in early age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252:1273–1281. doi: 10.1007/s00417-014-2690-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.