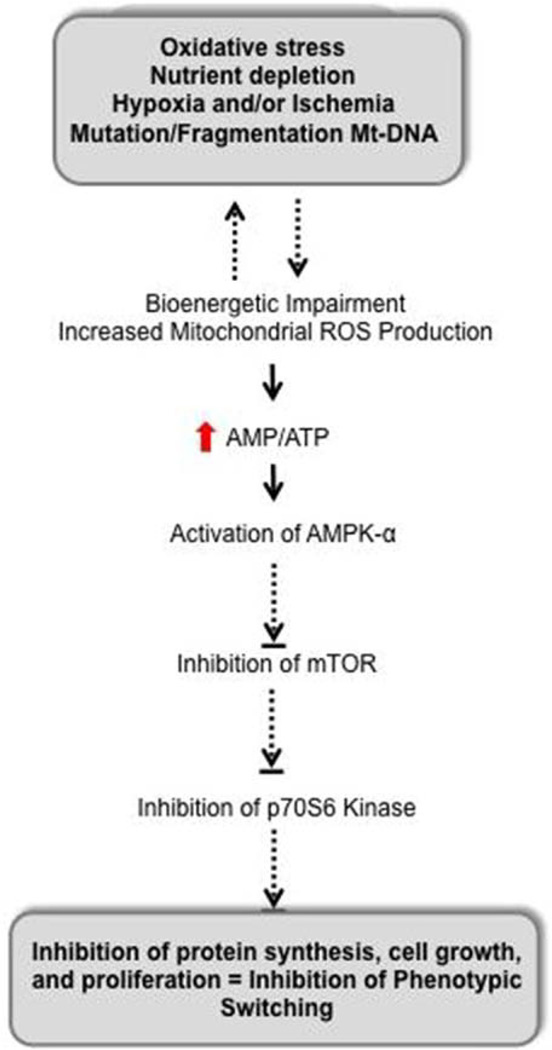
Figure 8.
Transduction of mitochondrial oxidative stress and dysfunction into impaired vascular growth. AMPK-α is a prototypical sensor of energy homeostasis, which is activated when cellular levels of AMP increase and ATP decreases. Activated AMPK then negatively regulates mammalian target of rapamycin (mTOR), which controls energy-demanding cellular processes, for example, protein synthesis, required for cell growth and proliferation. Thus, inhibition of mTOR signaling inhibits tube formation in human coronary artery endothelial cells, and we would also project that mitochondrial dysfunction in vivo would inhibit coronary collateral growth through similar mechanisms. Dephosphorylation/inactivation of p70S6 kinase, even in the presence of growth factor (ie, vascular endothelial growth factor), indicates that impaired mitochondrial bioenergetics would impair new protein synthesis. Thus, proper bioenergetics is critical to control interactions between phenotypic switching that requires new protein synthesis leading to vascular growth and cellular metabolism.