FIG 2.
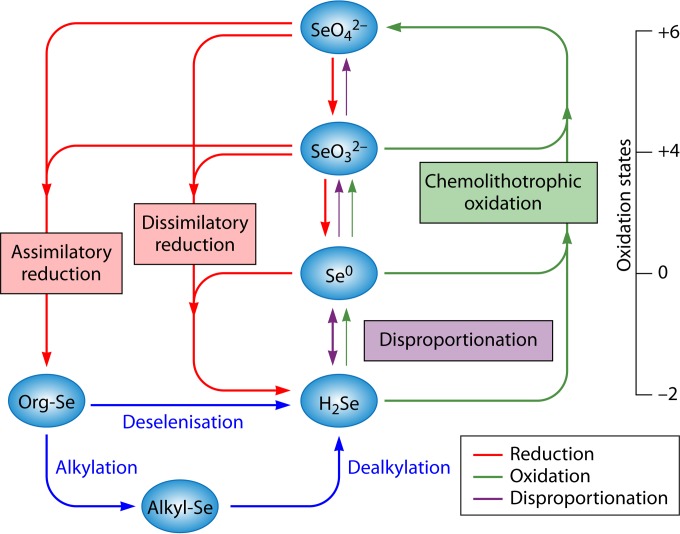
Selenium transformations in nature. Geologic and anthropogenic sources release selenium as SeO42− into the environment. SeRB are ubiquitous in natural settings and have a key role to play in the biochemical selenium cycle. SeRB have the ability to use selenate and/or selenite as a terminal electron acceptor in anaerobic respiration. The reduction of selenium oxyanions (SeO42− and SeO32−) is coupled to the degradation of organic matter in anaerobic sediments. Both SeO42− and SeO32− can be reduced to Se0 by bacteria under anaerobic conditions. Some bacteria reduce SeO42− and can incorporate it into organic compounds, i.e., selenoproteins. Reduction of SeO42− to Se0 appears to be a major sink for Se oxyanions in anoxic sediments. Reduction of Se(VI) or Se(IV) by SeRB (i.e., Sulfurospirillum barnesii and Bacillus arseniciselenatis) results in the production of insoluble elemental Se0. The opposite processes, such as oxidation of elemental selenium, can occur in soils and sediments, albeit at lower rates. Further reduction of elemental Se0 by other microorganisms (i.e., Bacillus selenitireducens) can lead to the formation of selenide. The selenide is then oxidized aerobically by selenium-oxidizing bacteria (SeOB), such as Thiobacillus, Thiothrix, etc. Alkylation reactions, which produce volatile (CH3)2Se and (CH3)2Se2, and dealkylation are also important processes mediated by microorganisms in soil and water (5, 9).