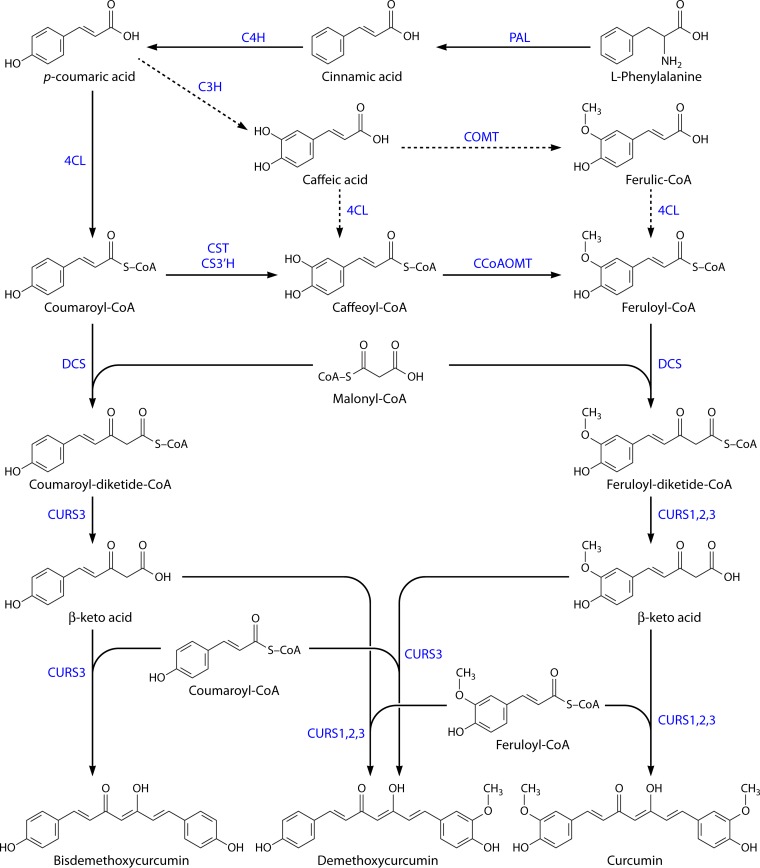
FIG 1.
Curcuminoid biosynthetic pathway in Curcuma longa. Cinnamic acid is synthesized from phenylalanine by phenylalanine ammonia lyase (PAL) and converted to coumaric acid by cinnamate-4-hydroxylase (C4H). Then, 4-coumarate-CoA ligase (4CL) converts coumaric acid to coumaroyl-CoA, and p-coumaroyl shikimate transferase (CST), p-coumaroyl 5-O-shikimate 3′-hydroxylase (CS3′H), and caffeoyl-CoA O-methyltransferase (CCoAOMT) convert it to feruloyl-CoA. Coumaroyl-CoA and feruloyl-CoA are then converted by diketide-CoA synthase (DCS) to diketide-CoAs by condensation with malonyl-CoA. In the end, curcumin synthases (CURSs) catalyze the formation of curcuminoids by condensing the diketide-CoAs with coumaroyl-CoA and feruloyl-CoA. Depending on the combination, different curcuminoids are produced, namely, bisdemethoxycurcumin, demethoxycurcumin, and curcumin. The route indicated by dashed arrows corresponds to a less central phenylpropanoid pathway and may not occur in vivo in C. longa.