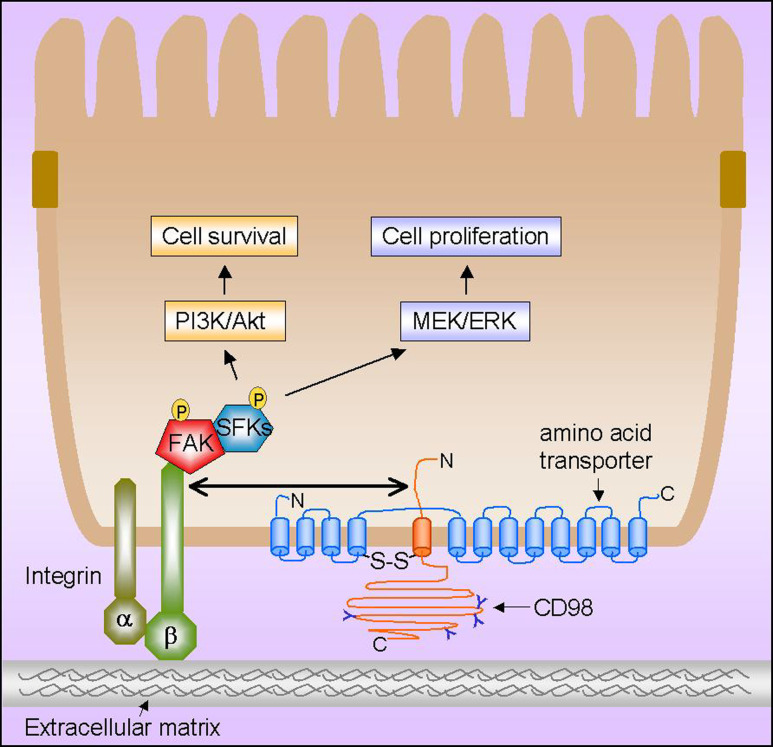
Fig. 1.
Schematic representation of the CD98-regulated cellular functions in intestinal epithelial cells mediated by interactions of CD98 with integrin and amino acid transporter. CD98 (heavy chain) with potential N-glycosylation sites (indicated as Y) is associated with an amino acid transporter (light chain) to form a functional heterodimer through a conserved disulphide bridge (S–S). The cytoplasmic tail of CD98 can interact with β1 and β3 integrin, thereby regulating integrin signaling. CD98-mediated integrin activation results in phosphorylation of the focal adhesion kinase (FAK), which functions as a phosphorylation-regulated scaffold to recruit Src-family kinases (SFKs) to focal adhesions. This consequently leads to activation of the phosphatidylinositol 3-kinase (PI3K)/Akt and mitogen-activated protein (MAP) kinase kinase (called MEK)/extracellular signal-regulated kinase (ERK) signaling pathways, promoting cell survival, proliferation, and migration