Abstract
Objective
This study aimed to retrospectively investigate the effect of dexmedetomidine on outcomes of patients undergoing CABG surgery.
Design
Retrospective investigation
Setting
Patients from a single tertiary medical center.
Participants
724 patients undergoing CABG surgery met the inclusion criteria were categorized into two groups: 345 in the dexmedetomidine group (DEX) and 379 in the non-dexmedetomidine group (Non-DEX).
Interventions
Perioperative dexmedetomidine use was defined as an intravenous infusion (0.24 to 0.6mcg/kg/hr) initiated after cardiopulmonary bypass and continued for less than 24 hours postoperatively in the intensive care unit.
Measurements and Main Results
Major outcome measures of this study were inhospital, 30-day and 1-year all cause mortality, delirium and major adverse cardiocerebral events. Perioperative dexmedetomidine infusion was associated with significant reduction in in-hospital, 30-day and 1-year mortalities, compared to the patients who did not received dexmedetomidine. In-hospital, 30-day and 1-year mortalities were 1.5% and 4.0% (adjusted Odds Ratio [OR], 0.332; 95% CI, 0.155 to 0.708; p = 0.0044), 2.0% and 4.5% (adjusted OR, 0.487; 95%CI, 0.253 to 0.985; p = 0.0305), 3.2% and 6.9% (adjusted OR 0.421; 95%CI, 0.247 to 0.718, p = 0.0015). Perioperative dexmedetomidine infusion was associated with a reduced risk of delirium from 4.6% to 7.9% (adjusted OR, 0.431; 95% CI, 0.265–0.701; P= 0.0007).
Conclusion
Dexmedetomidine infusion during CABG surgery was more likely to achieve improved in-hospital, 30-day and 1-year survival rates, and a significant lower incidence of delirium.
Keywords: CABG, Dexmedetomidine, Outcomes, Delirium
INTRODUCTION
Coronary artery disease (CAD) continues to be the leading cause of morbidity and mortality in the United States. Although epidemiologic evidence suggests that there has been a reduction in death attributable to coronary heart disease in recent years, it still has a considerably high morbidity and mortality rates.1 The 30-day mortality was about 1.2% in the on-pump coronary artery bypass graft (CABG) surgery, and in elderly patients (>65 years) mortality was 8.1% at 1 year. 1, 2 The major complications include postoperative delirium, infection, acute renal failure (ARF), and major adverse cardiocerebral events that include permanent or transient stroke, coma, perioperative myocardial infarction (MI), heart block and cardiac arrest.3 Although there are many contributing factors to these adverse events, surgical stress is one of the most important factors in the pathogenesis of these complications. Surgical stimuli cause increased plasma levels of epinephrine and norepinephrine and resulting in myocardial oxygen supply demand imbalance and myocardial ischemia, especially in patients with a compromised coronary blood flow.4, 5
Dexmedetomidine is a highly selective, shorter-acting intravenous alpha-2 agonist with 10-fold greater alpha-2 to alpha-1 receptor selectivity than clonidine.6 Intraoperative intravenous infusion of dexmedetomidine to patients undergoing CABG and vascular surgery decreased intraoperative sympathetic tone attenuated hyper-dynamic response and improved patient perioperative hemodynamics.7, 8 Laboratory studies have found that dexmedetomidine has a more profound protection against ischemia/reperfusion (I/R) and other forms of injuries in the heart, brain, kidneys, liver and lungs. 9–12 Moreover, dexmedetomidine has been shown to have anti-inflammatory and anti-delirium properties.13, 14 Our previous study found that perioperative dexmedetomidine use could reduced mortality in patients undergoing cardiac surgery, but there were no significant effect on the incidence of acute cardiac events.15 However, studies demonstrated that other alpha-2 agonists (Mivazerol and clonidine) significant reduced the incidence of cardiac events in non-cardiac patients with coronary artery disease. 16,17 Therefore, the specific aim of this study was to investigate the effect of dexmedetomidine on outcomes that include mortality and the postoperative complications of patients underwent CABG surgery only as a subgroup analysis.
METHODS
Study Design
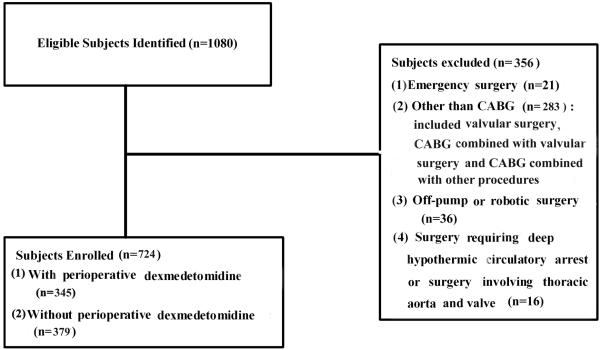
This study was a single center, retrospective and cohort study involving 1,080 consecutive patients who received cardiac surgery at a university medical center from January 1, 2006 to December 31, 2011. The study was reviewed and approved by the Institutional Review Board. The inclusion criteria were patients underwent CABG surgery only. The patients excluded from this study were those who underwent valvular surgery, combined CABG with other procedures, surgery involved aorta or any cardiac surgeries other than CABG surgery. Patients with emergency surgery, surgeries required deep hypothermia circulatory arrest, off-pump, robotic surgery and redo CABG surgery were also excluded from this study (Figure 1). Of all patients, 724 patients met the inclusion criteria with an average age of 66 years old. They were divided into two groups: using dexmedetomidine (DEX group, n=345, 47.65%) or not using dexmedetomidine (Non-DEX group, n=379, 52.35%) during the perioperative period (Figure 1).
Figure 1.
Recruiting of study samples.
Data Collection
The patient data were collected and organized to follow the template of the Society of Thoracic Surgeons (STS) National Database and patient hospital medical records, including demographics, patient medical history, preoperative risk factors, preoperative medications, intraoperative data, postoperative stroke, coma, MI, heart block and cardiac arrest, ARF, sepsis, delirium, and in-hospital, 30-day and 1-year all cause mortality. Independent investigators prospectively collected the data on each patient during the course of hospitalization for CABG surgery. General anesthesia was induced with midazolam, propofol or etomidate, fentanyl, lidocaine, and rocuronium. Anesthesia was maintained with oxygen and sevoflurane. Ventilation was controlled to an end tidal CO2 of 30–40 mmHg by adjusting tidal volume and/or respiratory rate. A right radial arterial catheter was placed for continuous blood pressure monitoring. A pulmonary artery catheter was placed via the right/left internal jugular vein. Transesophargeal echocardiography(TEE) was used in all cases for assessing cardiac function during surgery. Dexmedetomidine use was defined as intravenous infusion of this medication at a dose of 0.24–0.6 mcg/kg/hour after cardiopulmonary bypass (CPB) and continued for less than 24 hours postoperatively in the intensive care unit (ICU).
Major outcomes of this study were in-hospital, 30-day and 1-year all cause mortality, MI, postoperative RF/dialysis requirement, stroke, coma, delirium, heart block and cardiac arrest. Other outcomes were 30-day readmission, postoperative length of ICU stay and postoperative length of hospital stay (LOS). Based on the STS criteria, permanent stroke is defined as a postoperative stroke of any confirmed neurological deficit of abrupt onset caused by a disturbance in cerebral blood supply that did not resolve within 24 hours. Delirium indicates whether the patient experienced delirium tremens in the postoperative period marked by illusions, confusion, cerebral excitement, and having a comparatively short course. MI is documented by the following criteria (< 24 hours post-op): the creatine phosphokinase-MB (CK-MB), must be greater than or equal to 5 times the upper limit of normal, with or without new Q waves present in two or more contiguous electrocardiograph (ECG) leads, no symptoms required; or as documented by at least one of the following criteria (> 24 hours post-op): 1) evolutionary ST- segment elevations, 2) development of new Q waves in two or more contiguous ECG leads, 3) new or presumably new left bundle branch block pattern on the ECG, 4) the CK-MB must be greater than or equal to 3 times the upper limit of normal; heart block as a new heart block requiring the implantation of a permanent pacemaker of any type prior to discharge; postoperative RF as acute or worsening renal failure resulting in one or more of the followings: increase in serum creatinine >2.0 mg/dL and 2x most recent preoperative creatinine level over baseline or new requirement for dialysis postoperatively. Patients diagnosed as sepsis required positive blood cultures in the post-operative period. Any complication includes postoperative events occurred during the hospitalization for surgery. This includes the entire postoperative period up to discharge, even if over 30 days. The remaining definitions are available at http://www.sts.org/documents/pdf/trainingmanuals/adult2.73/V-c-AdultCVDataSpecifications2.73.pdf (accessed at June 30, 2012).
Statistical Analysis
Continuous and categorical variables were reported as mean ± SD or percentages, and compared with the t tests or chi-square test (two tailed), respectively. Univariate and multivariate logistic regressions were performed to assess associations of demographic, therapeutic and clinical outcome variables. To mitigate selection bias in dexmedetomidine infusion, we computed the propensity score, that is, the conditional probability of each patient receiving dexmedetomidine with a multivariable logistic regression model that includes patient demographic and clinical risk factors (Table 1, Supplemental Figure).
Table 1.
. Demographic and clinical characteristics
| Characteristics | Dexmedetomidine | p-value | |
|---|---|---|---|
| Yes (N=345) | No (N=379) | ||
| Age | 62.9±11.8 | 64.1±11.4 | 0.1694 |
| Gender(F) | 92(26.7) | 108(28.5) | 0.5827 |
| Race (White) | 118(34.2) | 118(31.1) | 0.3794 |
| BMI | 29.4±6.1 | 29.8±7.0 | 0.4665 |
| Hemoglobin (g/dl) | 14.9±2.8 | 15.1±3.1 | 0.5322 |
| Past medical history | |||
| Smoking | 185(53.6) | 208(54.9) | 0.7345 |
| Current Smoking | 70(20.3) | 77(20.3) | 0.9929 |
| Chronic lung disease | 48(13.9) | 57(15.0) | 0.6380 |
| Cerebrovascular Disease | 57(16.5) | 69(18.2) | 0.8701 |
| Peripheral Vascular Disease | 45(13.0) | 51(13.5) | 0.8701 |
| Family History CAD | 65(18.8) | 103(27.2) | 0.0080 |
| Diabetes | 129(37.4) | 113(29.8) | 0.031 |
| Hypertension | 271(78.6) | 289(76.3) | 0.4611 |
| Hypercholesterolemia | 241(69.9) | 304(80.2) | 0.0013 |
| Dyslipidemia | 233(67.5) | 158(41.7) | <0.0001 |
| History of Renal Failure | 17(4.9) | 8(2.1) | 0.0383 |
| Dialysis | 12(3.5) | 7(1.9) | 0.1706 |
| Pre-op Last Creatinine Level | 1.23±1.1 | 1.14±0.68 | 0.1982 |
| Pre-op MI | 121(35.1) | 167(44.1) | 0.0136 |
| CHF | 104(30.1) | 27(7.1) | <0.0001 |
| EF% | 49.7 ±13.3 | 51.9 ±13.0 | 0.0219 |
| Preoperative Medication | |||
| ACEi | 165(47.8) | 220(58.1) | 0.0059 |
| Beta Blockers | 242(70.10) | 255(67.30) | 0.4073 |
| ADP Inhibitors | 19(5.5) | 45(11.9) | 0.0026 |
| Nitrates | 11(3.2) | 13(3. 4) | 0.8561 |
| Anti platelets | 6(1.7) | 4(1.1) | 0.4314 |
| Anticoagulants | 65(18.8) | 92(24.3) | 0.0024 |
| Coumadin | 26(7.5) | 26(6.9) | 0.7251 |
| Inotropes | 2(0.6) | 4(1.1) | 0.4810 |
| Aspirin | 270(78.3) | 305(80.7) | 0.4093 |
| Lipid Lowering Medications | 138(40.0) | 252(66.5) | <0.0001 |
| GPIIb/IIIa Inhibitors | 8(2.3) | 24(6.3) | 0.0087 |
| Propensity score | 0.38(0.22) | 0.63(0.22) | P<0.0001 |
Values are n (%) for categorical variables and mean±SD for continuous variables. BMI, Body Mass Index; CAD, Coronary Arterial Disease; Pre-op MI, preoperative myocardial infarction (MI); CHF, Chronic heart failure; EF, ejection fraction; ACEi, angiotensin converting enzyme inhibitors. ADP, Adenosine diphosphate; GPIIb/IIIa Inhibitors, glycoprotein IIb/IIIa inhibitors
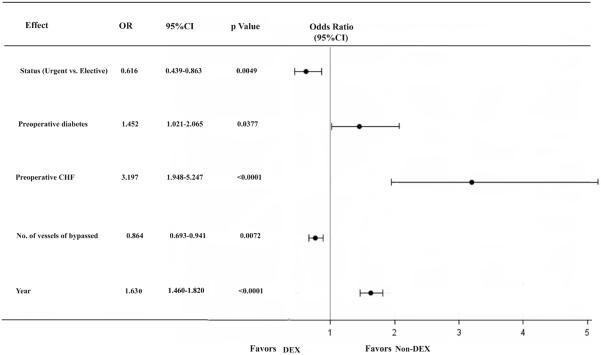
To achieve model parsimony and stability, the backward selection procedure was applied with the dropout criterion P > 0.05. The candidate risk factors were selected on the basis of the literature reviews, clinical plausibility, and variables collected in the database. The candidate independent variables included demographic and clinical risk factors (Table 1). The parsimonious multivariable propensity final model for dexmedetomidine use included status of procedure, preoperative diabetes, preoperative CHF, number of vessels bypassed and year of surgery (Figure 2). The risk-adjusted odds ratios for all outcomes were calculated with the use of a stepwise logistic-regression model with patient risk factors as independent control variables and dexmedetomidine use included in the model as the independent study variable of interest. A propensity-weighted logistic regression model was used for 1-year mortality in which an inverse (estimated) propensity score as weights for patients with dexmedetomidine and the inverse of 1 minus the propensity score for patients without dexmedetomidine and added dexmedetomidine as an independent factor to the model. All models fit analysis was evaluated with the Hosmer-Lemeshow goodness-of-fit statistic. The C statistic was reported as a measure of predictive power. Based on the propensity of dexmedetomidine use we classified all patients into quintile where quintile 1 contained patients with lowest propensity scores and quintile 5 contained patients with the highest propensity scores. Then, with a general linear model, we compared the propensity weighted and risk adjusted 1-year mortality between the cohort of dexmedetomidine used and the cohort of no dexmedetomidine used for each propensity-matched quintile. Results are reported as percentages and odds ratios (OR) and with 95% confidence intervals (CI). The model was calibrated among deciles of observed and expected risks for 1-year mortality (Hosmer-Lemeshow χ2: 12.018, c= 0.781, P =0.1504), dexmedetomidine use (Hosmer-Lemeshow χ2: 15.2236; c=0.785, P =0.0549).
Figure 2.
Parsimonious multivariable propensity model for dexmedetomidine use. OR, odds ratio; CI, confidence interval; CHF, congestive heart failure; DEX, dexmedetomidine
Furthermore, we performed survival analysis and presented Kaplan-Meier curves for patients in DEX group vs. patients in Non-DEX group. A parsimonious Cox proportional hazards model was created to evaluate the effect of dexmedetomidine for 1-year survival. All reported p values were 2-sided and p values < 0.05 were considered to be statistically significant. Statistical analysis was performed with SAS version 9.3 for Windows (SAS Inc., Cary, NC).
RESULTS
Baseline and intraoperative parameters
Demographic and clinical data of the patients who did and did not receive perioperative dexmedetomidine therapy are presented in Table 1. Patients in the DEX group presented more often with a history of CHF, low EF, RF, dyslipidemia and diabetes. Patients in the DEX group also presented more with preoperative use of beta-blockers and lipid lowering medications. However, patients in the Non-DEX group presented more often with a history of family History CAD, hypercholesterolemia and preoperative MI. Patients in the Non-DEX group also presented more with preoperative use of angiotensin converting enzyme inhibitors (ACEi), adenosine diphosphate (ADP) inhibitors, anticoagulants and glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors.
Procedural Characteristics
Procedural characteristics, including the number of vessels bypassed, IABP use were similar in both groups. In contrast, perfusion time (185.5 ±777 vs. 197.7 ±82.2, p=0.0106) and aortic cross-clamp time (129.3 ±63.6 vs.143.0±60.9, p=0.003) were significantly longer in Non-DEX group (Table 2).
Table 2.
. Procedural Characteristics
| Characteristics | Dexmedetomidine |
p-value | |
|---|---|---|---|
| Yes (N=345) | No (N=379) | ||
| Perfusion Time (min) | 182.5 ±77.7 | 197.7 ±82.2 | 0.0106 |
| IABP used, no. (%) | 28(8. 1) | 43(11.4) | 0.1447 |
| Cross Clamp Time (min) | 129.3 ±63.6 | 143.0±60.9 | 0.0030 |
| No. of vessels bypassed | 3.92±1.16 | 4.01±1.08 | 0.337 |
| No. of Vessels bypassed (>4) | 233(67.5) | 264(69.7) | 0.539 |
| Surgery, no | |||
| 2006 | 32(9.28) | 81(21.37) | 0.0112 |
| 2007 | 52(15.07) | 68(17.94) | 0.0793 |
| 2008 | 63(18.26) | 55(14.51) | 0.1027 |
| 2009 | 52(15.07) | 69(18.21) | 0.07351 |
| 2010 | 69(20.0) | 63(16.62) | 0.2141 |
| 2011 | 77(22.32) | 43(11.35) | 0.03265 |
| Surgeon, no/no. (%) | |||
| #1 | 270/343 (78.7) | ||
| #2 | 129/154 (83.7) | ||
| #3 | 203/247(82.2) | ||
| #4 | 122/144 (84.7) | ||
Values are n (%) for categorical variables and mean ± SD for continuous variables. IABP, intra-aortic balloon pump; no/no, number of enrolled patients/total numbers of cardiac surgeries performed by one specific surgeon
Postoperative complications and mortality
Univariate Analysis
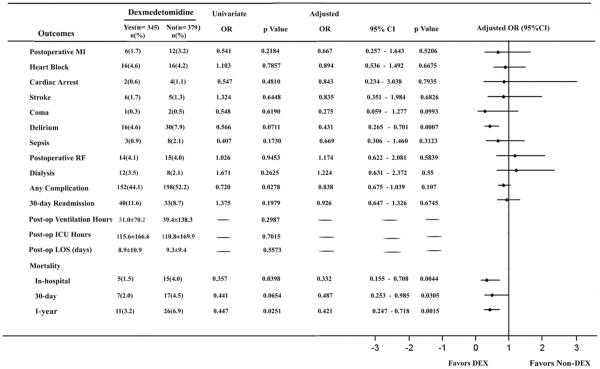
Perioperative infusion of dexmedetomidine was associated with significantly reduced in in-hospital and 1-year mortalities, but not in 30-day mortality. In-hospital mortality was 1.5% in the DEX group vs. 4.0% in the Non- DEX group (OR, 0.357; 95% CI, 0.128 to 0.993; p = 0.0398). 1-year mortality was 3.2% in the DEX group vs. 6.9% in the Non-DEX group (OR, 0.447; 95%CI, 0.218 to 0.919; p = 0.0251) (Figure 3). The perioperative use of dexmedetomidine was associated with a significantly reduced incidence of any complication (44.1% vs. 52.20%, OR, 0.720; 95% CI, 0.537 to 0.965; p= 0.0278) (Figure 3).
Figure 3.
Effects of dexmedetomidine on postoperative complications and mortality in patients undergoing CABG surgery. Values are numbers (%) for categorical variables and mean± SD for continuous variables. OR, odd ratio; CI, confidence interval; MI, myocardial infarction; RF, renal failure; Post-op, post-operative; ICU, Intensive care unit; LOS, length of stay; DEX, dexmedetomidine.
Propensity and Multivariate Analysis
The risk-adjusted results for all outcomes are summarized in Figure 3. The observed reduction in in-hospital (adjusted OR, 0.332; 95% CI, 0.155 to 0.708; p=0.0044) and 1-year (adjusted OR, 0.421; 95% CI, 0.247 to 0.718; p=0.0015) mortalities in patients receiving perioperative dexmedetomidine remained to be significant after propensity adjustment. 30-day mortality (adjusted OR, 0.487; 95% CI, 0.253 to 0.985; p=0.0305) reduced significant in patients receiving perioperative dexmedetomidine after adjustment. The adjusted rates of delirium (adjusted OR, 0.431; 95% CI, 0.265 to 0.701; p= 0.0007) were also statistically significant between the DEX and Non-DEX groups. However, there were no statistical differences in the incidence of any complication between groups after adjusting for differences between groups, although the OR point estimates favor perioperative dexmedetomidine use (Figure 3).
1-year Mortality and Survival Analysis
Patients who received dexmedetomidine in all five quintiles were significantly lower with respect to 1-year mortality compared to the patients in the Non-DEX group (Table 3).
Table 3.
Predicted 1-Year Mortality by Quintile of Propensity Score
| Quintile | Number | Propensity score | p-valve | Mortality | p-valve | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| DEX | Non-DEX | DEX | Non-DEX | DEX | Non-DEX | |||
| 1 | 17 | 127 | 0.180 | 0.158 | 0.7964 | 0.0266 | 0.08051 | 0.0022 |
| 2 | 46 | 98 | 0.305 | 0.300 | 0.999 | 0.0357 | 0.0798 | 0.0005 |
| 3 | 63 | 83 | 0.453 | 0.449 | 1 | 0.0305 | 0.0711 | 0.0007 |
| 4 | 104 | 41 | 0.632 | 0.629 | 1 | 0.0234 | 0.0703 | 0.0003 |
| 5 | 115 | 30 | 0.837 | 0.832 | 1 | 0.0250 | 0.0673 | 0.0092 |
Propensity score reflected mean propensity score; Mortality reflected mean predicted 1-year mortality; Quintile 1 contains patients with lowest propensity scores and quintile 5 contains patients with the highest propensity scores. DEX, dexmedetomidine.
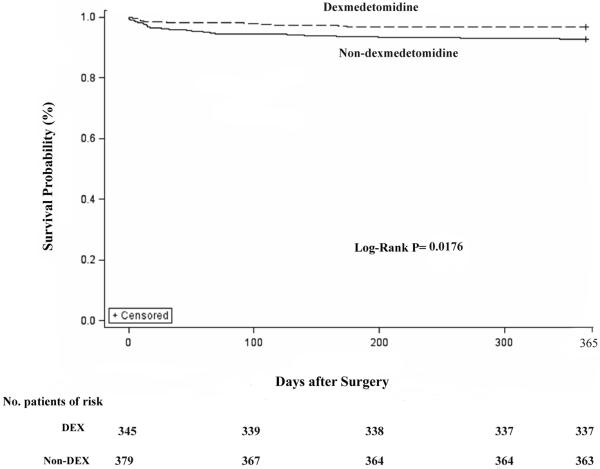
Survival probability was calculated using Kaplan-Meier methods and compared with the use of a Log-rank test (Chi-Square=5.6312, p=0.0176). For the duration of one year, there were significant differences in survival between the DEX and Non-DEX groups (propensity adjusted: 97.28% vs.92.23%, OR 0.421, 95%CI 0.247 to 0.718, p=0.00115) (Figure 4).
Figure 4.
Survival estimates after CABG surgery between two groups. Survival probability were calculated with the use of Kaplan-Meier methods and compared with the use of a Log-rank test (Log -Rank test, Chi-Square=5.6312, p=0.0176). DEX group, using dexmedetomidine after weaning off bypass; Non-DEX, not using dexmedetomidine after weaning off bypass.
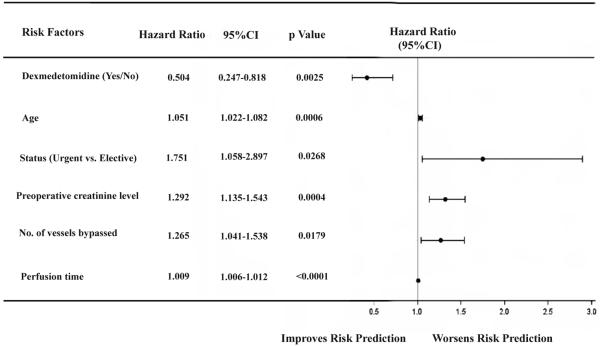
After risk adjustment, a Cox proportional hazard model analysis revealed that older patients (age>65 years), urgent surgery, preoperative last creatinine level, number of vessels bypassed and perfusion time significantly increase the 1-year mortality, whereas perioperative dexmedetomidine infusion significantly reduced the risk of death during the first year (Hazard ratio, 50.4%; 95% CI, 0.247 to 0.818; p=0.0025) compared to those in Non-DEX group at any time within one year after CABG surgery (Figure 5).
Figure 5.
Cox proportional hazard model for 1-year mortality following cardiac surgery. Values are numbers (%) for categorical variables and mean± SD for continuous variables. CI, confidence interval
DISCUSSION
This study is the first to demonstrate that dexmedetomidine administered during CABG surgery improved in-hospital, 30-day and 1-year survivals. Our results also suggest that perioperative dexmedetomidine use reduced postoperative incidence of delirium.
CAD continues to be the leading cause of morbidity and mortality in the United States. CABG is a main treatment procedure, but comes with a considerable high morbidity and mortality.1 Approximately 500,000 CABG surgeries were performed annually in the United States.18 The most common postoperative complications are including permanent or transient stroke, coma, perioperative MI, heart block and cardiac arrest, ARF, central nerve system (CNS) dysfunction and infections. The pathogenesis of these complications is multifactorial and involves surgical stress, systemic inflammatory response syndrome (SIRS) and I/R injury in patients undergoing on-pump CABG.
Cardiocerebral complications are often presented immediately after cardiac surgery, including stroke (1.4%–4.6%), cardiac arrest (0.7–2.9%) and MI (3.1%–9.1%).3,19–21 Although this study demonstrated that the risk of postoperative cardiocerebral events include MI, heart block, cardiac arrest, stroke and coma appeared no significant decrease during perioperative use of dexmedetomidine after CABG surgery, the OR values of these events are all in favor perioperative use of dexmedetomidine. Studies have demonstrated the effectiveness of dexmedetomidine as a stress suppressing, anti-inflammatory and anti- I/R injury agent in the prevention and treatment of cardiovascular events.6, 7 Intraoperative intravenous infusion of dexmedetomidine in patients undergoing CABG surgery decreased plasma norepinephrine (NE) concentration, and reduced intraoperative and postoperative episodes of hypertension and tachycardia.6 In an animal study, dexmedetomidine reduced serum catecholamine, heart rate and contractility, increased coronary blood flow and decreased myocardial oxygen consumption.22
Delirium is a common complication and increases morbidity and mortality in older ICU patients with a prevalence of 20–50% after cardiac surgery.23–25 The prevalence of delirium in our study was only 6.25% after CABG surgery because we only analyzed patients with hyperactive delirium. However, the major type of delirium is emotional (hypoactive) delirium instead of hyperactive delirium. Peterson and colleagues recently reported that the incidence of hyperactive delirium was only 1.6% in medical ICU patients whereas the majority of them had either a mixed (54.9%) or hypoactive (43.5%) delirium.26 Dexmedetomidine has been reported to reduce the incidence of delirium and its duration in septic and cardiac patients.27, 28 It has also been reported being used to treat ICU associated delirious agitation.29 Our study also confirmed that the incidence of delirium was significantly lower in the patients who received dexmedetomidine. Although the causes of delirium remain hypothetical, the abnormalities in neurotransmission, inflammation and anesthetic agents are among the leading causes to this brain dysfunction.30–32
This study demonstrated the in-hospital, 30-day and 1-year mortalities were significantly lower in patients who received dexmedetomidine. It reduced the risk of death by 49.6% during the first year. In a Cox proportional hazard model for 1-year mortality, age, urgent surgery, preoperative creatinine level, number of vessels bypassed and perfusion time appeared to be adversely affect 1-year mortality. The mechanism of the reduction of mortalities is likely associated with sympatholytic, anti-inflammatory and anti-delirium effects. Therefore, we believe the reduction of mortality represented the overall effects of dexmedetomidine.
This investigation has some limitations. In retrospective study, although multivariate regression in combination with the propensity score adjustment was applied to reduce evident biases, the potential confounding variables following a non-randomized study may not have been removed completely. The timing of dexmedetomidine use in this study was after CPB and lasted up to 24 hour postoperatively, which is different from other report which showed dexmedetomidine administration had a protection against I/R injury only before ischemia.33 However, the peak intraoperative plasma concentrations of norepinephrine and epinephrine occurred after CPB.34
Conclusions
This study is the first to demonstrate that on-pump CABG surgery patients who received the intravenous dexmedetomidine infusion during surgery were more likely to have better in-hospital, 30-day and 1-year survival rates after surgery. Perioperative use of dexmedetomidine is also associated with a significantly lower incidence of postoperative delirium. However, a well-designed, prospective, multicenter randomized study is needed to focus on the use of dexmedetomidine on CABG surgery patients to confirm these findings.
Supplementary Material
Acknowledgments
The authors wish to thank Sharon McKee for providing assistant in artwork and English editing.
Funding/Support This work was supported by the University of California Davis Health System Department of Anesthesiology and Pain Medicine, Department of Surgery and Department of Internal Medicine and NIH grant UL1 TR000002. This study was supported by a grant from Jiangsu Province's by Key Provincial Talents Program, China (Fuhai Ji), by Jiangsu province's six major peak talents program, China (Fuhai Ji) and by Suzhou science and No.SYS201111 (Fuhai Ji) from Technology Bureau's program, China.
Footnotes
Disclosures
None
ReferenceS
- 1.Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–1497. doi: 10.1056/NEJMoa1200388. [DOI] [PubMed] [Google Scholar]
- 2.Shahian DM, O'Brien SM, Sheng S, et al. Predictors of Long-Term Survival After Coronary Artery Bypass Grafting Surgery-Results From the Society of Thoracic Surgeons Adult Cardiac Surgery Database (The ASCERT Study) Circulation. 2012;125:1491–500. doi: 10.1161/CIRCULATIONAHA.111.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown PP, Kugelmass AD, Cohen DJ, et al. The frequency and cost of complications associated with coronary artery bypass grafting surgery: results from the United States Medicare program. Ann Thorac Surg. 2008;85:1980–1986. doi: 10.1016/j.athoracsur.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 4.Udelsman R, Norton JA, Jelenich SE, et al. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab. 1987;64:986–994. doi: 10.1210/jcem-64-5-986. [DOI] [PubMed] [Google Scholar]
- 5.Dawood MM, Gutpa DK, Southern J, et al. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. International Journal of Cardiology. 1996;57:37–44. doi: 10.1016/s0167-5273(96)02769-6. [DOI] [PubMed] [Google Scholar]
- 6.Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 7.Jalonen J, Hynynen M, Kuitunen A, et al. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology. 1997;86:331–345. doi: 10.1097/00000542-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Talke P, Li J, Jain U, et al. Effects of perioperative dexmedetomidine infusion in patients undergoing vascular surgery. Anesthesiology. 1995;82:620–633. doi: 10.1097/00000542-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Chih-Lin Yang, Cay-Huyen Chen, Pei-Shan Tsai, et al. Protective Effects of Dexmedetomidine-Ketamine Combination against ventilator-induced lung injury in Endotoxemia Rats. Journal of Surgical Research. 2011;167:e273–281. doi: 10.1016/j.jss.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Jianteng Gu, Pamela Sun, Hailin Zhao, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Critical Care. 2011;15:R153. doi: 10.1186/cc10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders RD, Xu J, Shu Y, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 12.Okada H, Kurita T, Mochizuki T, et al. The cardioprotective effect of dexmedetomidine on global ischaemia in isolated rat hearts. Resuscitation. 2007;74:538–545. doi: 10.1016/j.resuscitation.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi T, Kurita A, Kobayashi K, et al. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22:221–228. doi: 10.1007/s00540-008-0611-9. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50:206–217. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 15.Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127:1576–84. doi: 10.1161/CIRCULATIONAHA.112.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver MF, Goldman L, Julian DG, Holme I. Effect of mivazerol on perioperative cardiac complications during non-cardiac surgery in patients with coronary heart disease: the European Mivazerol Trial (EMIT) Anesthesiology. 2004;101:284–93. doi: 10.1097/00000542-199910000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Wallace AW, Galindez D, Salahieh A, et al. Effect of clonidine on cardiovascular morbidity and mortality after noncardiac surgery. Anesthesiology. 2004;101:284–93. doi: 10.1097/00000542-200408000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics-2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 19.Bucerius J, Gummert JF, Borger MA, et al. Stroke after cardiac surgery: a risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003;75:472–478. doi: 10.1016/s0003-4975(02)04370-9. [DOI] [PubMed] [Google Scholar]
- 20.Chen JC, Kaul P, Levy JH, et al. Myocardial infarction following coronary artery bypass graft surgery increases healthcare resource utilization. Crit Care Med. 2007;35:1296–1301. doi: 10.1097/01.CCM.0000262403.08546.A2. [DOI] [PubMed] [Google Scholar]
- 21.Mackay JH, Powell SJ, Osgathorp J, et al. Six year prospective audit of chest reopening after cardiac arrest. European Journal of Cardio-Thoracic Surgery. 2002;22:421–425. doi: 10.1016/s1010-7940(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence CJ, Prinzen FW, De Lange S. The effect of dexmedetomidine on the balance of myocardial energy requirement and oxygen supply and demand. Anesth Analg. 1996;82:544–550. doi: 10.1097/00000539-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Talke PO, Mangano DT. Alpha2-adrenergic agonists and perioperative ischemia. Anaesth Pharmacol Rev. 1993;1:310–315. [Google Scholar]
- 24.Inouye S. Current concepts, delirium in older persons. N Engl J Med. 2005;9:335–336. [Google Scholar]
- 25.Bakker RC, Osse RJ, Tulen JH, et al. Preoperative and operative predictors of delirium after cardiac surgery in elderly patients. Eur J Cardiothorac Surg. 2012;41:544–549. doi: 10.1093/ejcts/ezr031. [DOI] [PubMed] [Google Scholar]
- 26.Pandharipande P, Cotton Brya A, Shintani A, et al. Prevalence and Risk Factors for Development of Delirium in Surgical and Trauma Intensive Care Unit Patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009;111:1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 29.Reade MC, O'Sullivan K, Bates S, et al. Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: a randomised open-label trial. Crit Care. 2009;13:R75. doi: 10.1186/cc7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5:132–148. doi: 10.153/SCNP00500132. [DOI] [PubMed] [Google Scholar]
- 31.Simone MJ, Tan ZS. The role of inflammation in the pathogenesis of delirium and dementia in older adults: a review. CNS Neurosci Ther. 2011;17:506–513. doi: 10.1111/j.1755-5949.2010.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R, Kharbanda M, Sood N, et al. Comparative evaluation of incidence of emergence agitation and post-operative recovery profile in paediatric patients after isoflurane, sevoflurane and desflurane anaesthesia. Indian J Anaesth. 2012;56:156–161. doi: 10.4103/0019-5049.96325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulka PJ, Tryba M, Zenz M. Preoperative alpha2-adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery: results of a randomized, controlled trial. Crit Care Med. 1996;24:947–952. doi: 10.1097/00003246-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XY, Liu ZM, Wen SH, et al. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology. 2012;116:1035–1046. doi: 10.1097/ALN.0b013e3182503964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.