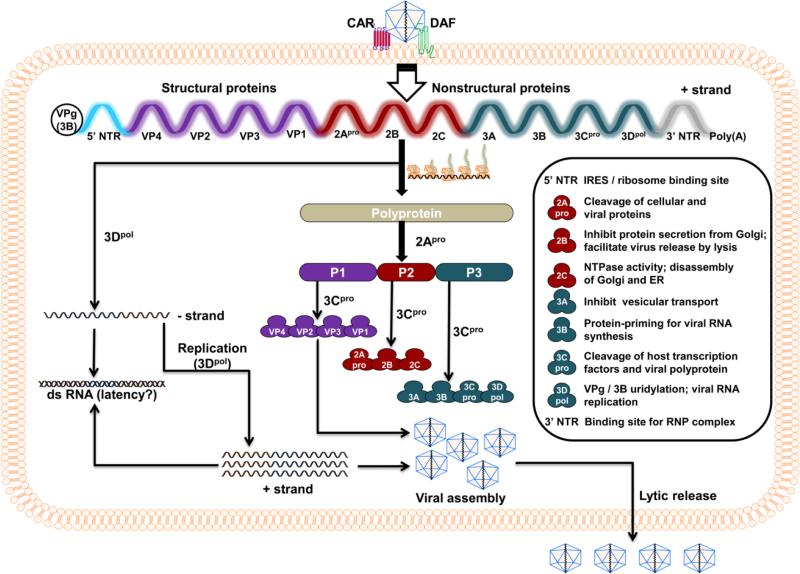
Fig. 1. The life cycle of CVB3.
Virus entry into the target cells first requires interaction with DAF, which facilitates viral attachment to CAR, leading to internalization of the virus in the cytoplasm. After uncoating, the positive-sense single-stranded RNA genome is translated to yield a large single polyprotein. This process requires binding of ribosomes to IRES. The polyprotein is then proteolytically cleaved to generate structural (P1 cluster: VP1 to VP4) and NS (P2 cluster: 2Apro, 2B and 2C; P3 cluster: 3A, 3B, 3Cpro and 3Dpol) proteins by 2Apro and 3Cpro, two viral proteases. While the structural proteins, also called capsid proteins, contribute to the conformation of the virus, NS proteins mediate a variety of functions as indicated in the inset. During viral replication, 3Dpol plays a critical role in the formation of negative and positive strands of viral genomes, which can be paired to form dsRNA as an intermediate stage. The progeny virus containing a single-strand positive-sense RNA genome and structural proteins is finally released through cell lysis.