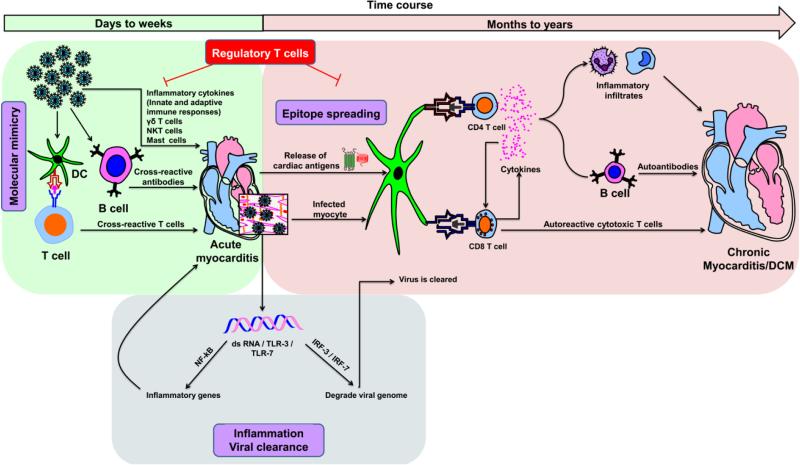
Fig. 3. Proposed mechanisms of inflammatory heart disease.
Acute myocarditis can result from an overt immune response to exposure to cardiotropic pathogens, and cardiac damage can be due to the production of inflammatory cytokines of innate and adaptive immune systems. On one hand, although transitory, dsRNAs produced during the replication cycle of CVB3 can bind TLR-3 or TLR-7 and promote inflammatory response through the activation of NFkB. On the other, dsRNAs can mediate viral clearance by degrading the viral genome by activating IRF-3 / IRF-7 pathways. Various innate immune cell types, such as γδ T cells, NK-T cells and mast cells, can influence the disease-outcome. While γδ T cells and mast cells promote cardiac damage, NK-T cells can dampen such a response. Exposure to environmental microbes that carry mimicry sequences for cardiac antigens can lead to the generation of pathogenic cross-reactive T cell and antibody responses. Nonetheless, once cardiac damage sets in, intracellular proteins (e.g., Myhc-α) that were previously invisible to the immune system can be released as a result of epitope spreading, leading to their uptake by APCs (e.g., DCs) and inducing CD4 T cell responses. Alternatively, APCs can engulf infected cardiomyocytes and induce both CD4 and/or CD8 T cell responses via cross-priming. Upon activation, antigen-sensitized CD4 T cells secrete Th1 and Th17 cytokines that favor heart autoimmunity by activating macrophages and promoting neutrophil infiltration, thereby aggravating cardiac damage while providing help to CD8 T cells and B cells via cytokines and chemokines. CD8 T cells can contribute to myocarditis in two ways: 1) secretion of cytokines similar to those secreted by CD4 T cells with identical consequences; and 2) killing of cardiomyocytes infected with cardiotropic pathogens (e.g., CVB3) via MHC class I-dependent pathway. In the meantime, pathogen-specific T cells, neutralizing antibodies and antiviral cytokines such as IFN-α, IFN-β can be generated, which facilitate elimination of the microbe. Nonetheless, the newly generated cardiac-reactive CD4 and CD8 T cells can persist and contribute to chronic inflammation. In these scenarios, however, presence of efficient Treg cells can dampen autoimmune responses, as they are highly critical for maintenance of self-tolerance.