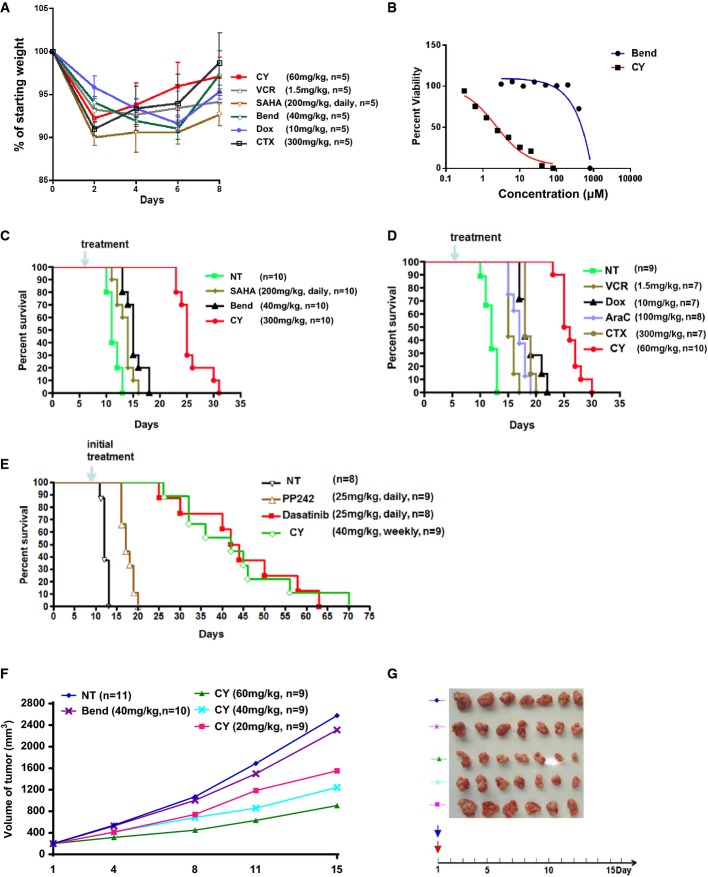
A Determination of maximally tolerated dose (MTD) of CY at 60 mg/kg. Other drugs were used at MTD according to the literature. For each drug, n = 5 and all mice survived treatment. Data represent mean ± SEM.
B CY exhibited enhanced activity against BCR-ABL Arf−/− murine ALL cells in vitro.
C, D CY showed superior antitumor activity compared with bendamustine, SAHA, and other chemotherapeutic drugs in mice transplanted with BCR-ABL ALL cells. P-values of CY versus other drugs: NT (P = 5.02E-06) (C), SAHA (P = 4.45E-06), Bend (P = 5.18E-06), NT (P = 1.20E-05) (D), VCR (P = 1.13E-05), Dox (P = 8.08E-06), AraC (P = 7.62E-06), and CTX (P = 1.13E-05). Survival statistical analysis was done with the Mantel–Cox (log-rank) test of GraphPad Prism.
E Therapeutic effects of CY (weekly dose) and targeted drugs PP242 (daily dose) and dasatinib (daily dose) in mice transplanted with BCL-ABL ALL cells. P-values of CY versus other drugs: NT (P = 3.94E-05), PP242 (P = 1.20E-05), and dasatinib (P = 0.99). Survival statistical analysis was done with the Mantel–Cox (log-rank) test of GraphPad Prism.
F, G Antitumor activity of CY and bendamustine in nude mice models. Mice were inoculated with human NSCLC cell line H460. When tumor volume reached 200 mm3, mice were treated with single dose of bendamustine (40 mg/kg, MTD) or CY (20, 40, 60 mg/kg). In (G), tumors were dissected out after 15 days posttreatment.