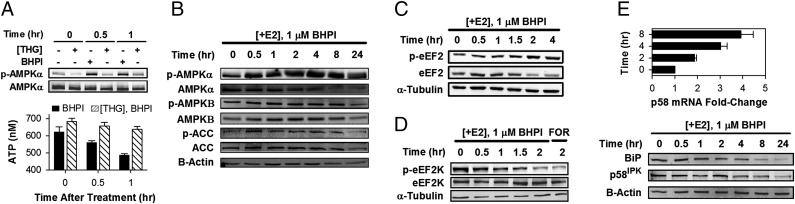
Fig. 5.
BHPI depletes intracellular ATP stores, activates AMPK, and inhibits protein synthesis at a second site. (A) Inhibiting SERCA pumps with thapsigargin (THG) prevents BHPI from reducing intracellular ATP levels. Western blot shows effect of THG (1 μM) or BHPI (1 μM) treatment of MCF-7 cells on AMPKα-Thr172 phosphorylation. ATP levels in MCF-7 cells were treated with 1 μM BHPI or 1 μM BHPI and 1 μM THG (n = 5). (B) Western blot analysis of the time course of AMPKα (Thr-172), AMPKβ (Ser-108), and acetyl CoA carboxylase (ACC) (Ser-79) phosphorylation in BHPI-treated MCF-7 cells. AMPKα-Thr172 and AMPKβ-Ser108 phosphorylation are required for AMPK activation. (C) Western blot analysis of eEF2 phosphorylation (Thr-56) over time in BHPI-treated ERα+ MCF-7 cells. (D) Western blot analysis showing the time course of decreasing eEF2K (Ser-366) phosphorylation in BHPI-treated MCF-7 cells. Ser-366 dephosphorylation activates eEF2K. (E) qRT-PCR analysis showing changes in p58IPK mRNA and Western blot analysis showing p58IPK and BiP protein after treatment with BHPI (n = 3). −E2 set to 1.