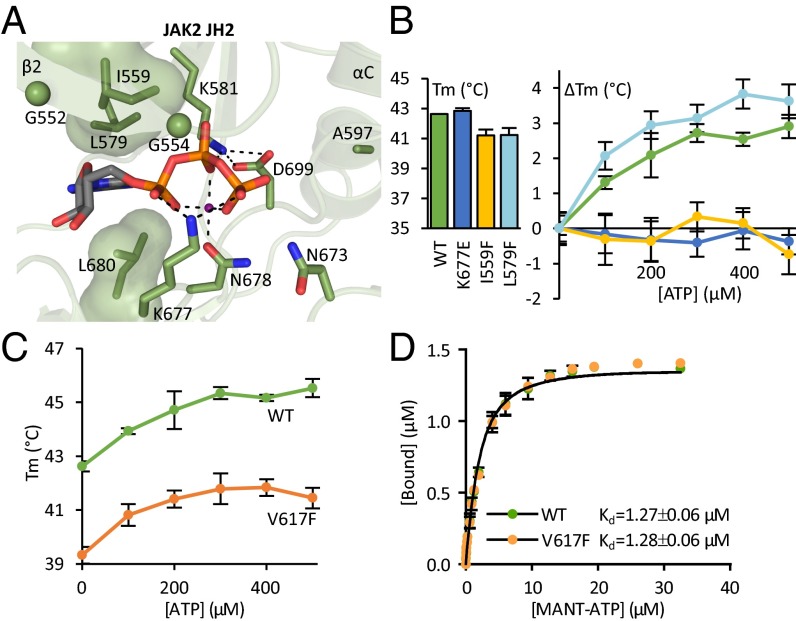
Fig. 1.
Characterizing the ATP binding pocket of JAK2 JH2. (A) The ATP binding pocket of JAK2 JH2 (16) [Protein Data Bank (PDB) ID code 4FVQ] highlighting the noncanonical mode of nucleotide binding. JAK2 JH2 contains a bulky leucine (Leu579) on the N-lobe side of the purine pocket substituting for the canonical alanine in the VAIK motif. The glycine-rich loop consists of two glycines (shown as spheres) rather than three. The phosphates of bound ATP (shown as sticks) are coordinated by only one divalent cation (shown in magenta) instead of two in typical kinases. Furthermore, Asp699 of the DPG motif (consensus DFG) forms a salt bridge to the β3 lysine (Lys581), and Lys677 from the catalytic loop binds directly to the α and γ phosphates of ATP. Dotted lines highlight hydrogen bonds and salt bridges participating in the binding of ATP. The hydrophobic amino acids lining the purine base and sugar moiety binding site are shown as volume-filling models. (B) Fluorometric TSA of recombinant JAK2 JH2. Tms of JAK2 JH2 ATP binding site mutants are shown in the bar graph. TSA shows no thermal stabilization for JAK2 JH2 I559F or JAK2 JH2 K677E upon addition of ATP (ΔTm, line graph). (C) Thermal stability of recombinant JAK2 JH2 wild type and V617F upon addition of ATP to wild-type and V617F JH2 shows similar ATP responses yet overall reduced thermal stability in V617F. The data for wild type are the same as shown in B. (D) MANT-ATP binding assay on recombinant JAK2 JH2 reveals identical MANT-ATP binding affinities for wild type and V617F. All experiments were done in the presence of Mg2+. All error bars are standard deviations (SD) from triplicate experiments.