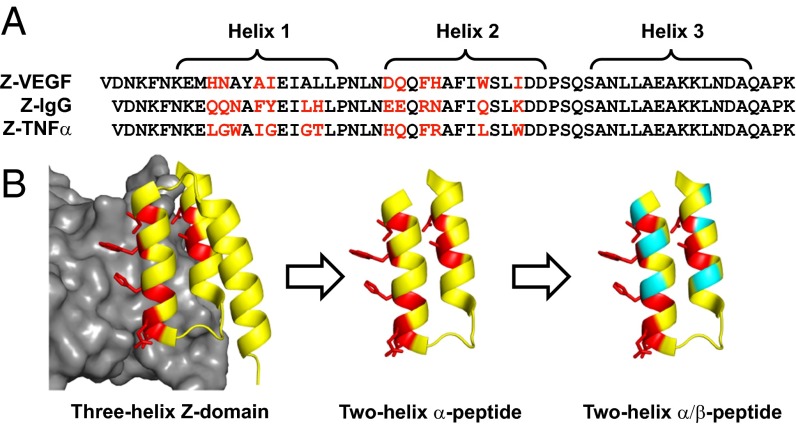
Fig. 1.
Design of α/β-peptides based on the Z-domain scaffold. (A) Sequences of peptides previously derived from the Z-domain scaffold Z-VEGF, Z-IgG, and Z-TNFα targeting VEGF (19), IgG (16), and TNFα (20), respectively. Helices 1, 2, and 3 are indicated by brackets. For Z-VEGF and Z-TNFα, residues on the protein-binding face of helices 1 and 2 that were identified via randomization and selection (including the unintentionally incorporated Ala14 in Z-VEGF) are shown in red. For Z-IgG, the parent Z-domain, red positions indicate the corresponding residues that contact IgG. Sequences are arranged based on structural alignment of helical regions. (B) Strategy for the design of α/β-peptide mimics of Z-VEGF (shown in yellow and red). Red residues indicate selected residues that contact VEGF8–109 (shown in gray) in the cocrystal structure. Sites targeted for nonnatural amino acid substitutions shown in teal. Figure is based on PDB ID code 3S1K.