Abstract
B cells proliferate and diversify in germinal centers in response to antigen. Only a small percentage of these B cells will emerge to form the serum antibody response. Other B cells making lower affinity antibodies, acquiring nonsense mutations, or expressing autoreactivity as a result of somatic mutation undergo an apoptotic cell death and are not efficiently sampled in current analyses of B-cell hybridomas. We have demonstrated that expression of bcl-2 in the NSO myeloma fusion partner leads to a higher yield of viable hybridomas, with a selective increase in hybridomas from B cells that produce autoantibodies and are seldom recovered when spleen cells from non-autoimmune mice are fused to the conventional NSO cell line. Using this fusion partner, we have generated hybridomas from anti-DNA antibody-producing transgenic B cells that are anergic in vivo and destined for apoptosis. These studies provide a strategy to sample the repertoire of B cells that arise in vivo but are not selected to contribute to the expressed antibody response. Furthermore, they demonstrate that restricted expression of bcl-2 in B cells contributes to the maintenance of self-tolerance in secondary lymphoid organs.
Full text
PDF

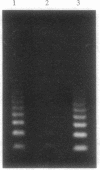
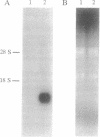
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berek C., Ziegner M. The maturation of the immune response. Immunol Today. 1993 Aug;14(8):400–404. doi: 10.1016/0167-5699(93)90143-9. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Cuende E., Alés-Martínez J. E., Ding L., Gónzalez-García M., Martínez C., Nunez G. Programmed cell death by bcl-2-dependent and independent mechanisms in B lymphoma cells. EMBO J. 1993 Apr;12(4):1555–1560. doi: 10.1002/j.1460-2075.1993.tb05799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrassi A., Hilbert D. M., Rudikoff S., Anderson A. O., Potter M., Coon H. G. In vitro culture of primary plasmacytomas requires stromal cell feeder layers. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2060–2064. doi: 10.1073/pnas.90.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond B., Katz J. B., Paul E., Aranow C., Lustgarten D., Scharff M. D. The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol. 1992;10:731–757. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- Diamond B., Scharff M. D. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung M. C., Wensink P. C. Different restriction enzyme-generated sticky DNA ends can be joined in vitro. Nucleic Acids Res. 1984 Feb 24;12(4):1863–1874. doi: 10.1093/nar/12.4.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Kluck R. M., Chapman D. E., Egan M., McDougall C. A., Harmon B. V., Moss D. J., Kerr J. F., Halliday J. W. Spontaneous apoptosis in NS-1 myeloma cultures: effects of cell density, conditioned medium and acid pH. Immunobiology. 1993 Jun;188(1-2):124–133. doi: 10.1016/S0171-2985(11)80492-4. [DOI] [PubMed] [Google Scholar]
- Kroese F. G., Wubbena A. S., Seijen H. G., Nieuwenhuis P. Germinal centers develop oligoclonally. Eur J Immunol. 1987 Jul;17(7):1069–1072. doi: 10.1002/eji.1830170726. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Mason D. Y., Johnson G. D., Abbot S., Gregory C. D., Hardie D. L., Gordon J., MacLennan I. C. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur J Immunol. 1991 Aug;21(8):1905–1910. doi: 10.1002/eji.1830210819. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Liu Y. J., Oldfield S., Zhang J., Lane P. J. The evolution of B-cell clones. Curr Top Microbiol Immunol. 1990;159:37–63. doi: 10.1007/978-3-642-75244-5_3. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis P., Opstelten D. Functional anatomy of germinal centers. Am J Anat. 1984 Jul;170(3):421–435. doi: 10.1002/aja.1001700315. [DOI] [PubMed] [Google Scholar]
- Nisitani S., Tsubata T., Murakami M., Okamoto M., Honjo T. The bcl-2 gene product inhibits clonal deletion of self-reactive B lymphocytes in the periphery but not in the bone marrow. J Exp Med. 1993 Oct 1;178(4):1247–1254. doi: 10.1084/jem.178.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez G., Hockenbery D., McDonnell T. J., Sorensen C. M., Korsmeyer S. J. Bcl-2 maintains B cell memory. Nature. 1991 Sep 5;353(6339):71–73. doi: 10.1038/353071a0. [DOI] [PubMed] [Google Scholar]
- Offen D., Spatz L., Escowitz H., Factor S., Diamond B. Induction of tolerance to an IgG autoantibody. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8332–8336. doi: 10.1073/pnas.89.17.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. F., Szalay M. G., Paskar L., Sahai B. M., Boyer M., Singh B., Green D. R. Activation-induced cell death in T cell hybridomas is due to apoptosis. Morphologic aspects and DNA fragmentation. J Immunol. 1990 May 1;144(9):3326–3333. [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Vaux D. L., Webb E., Bath M. L., Adams J. M., Cory S. Abnormalities of the immune system induced by dysregulated bcl-2 expression in transgenic mice. Curr Top Microbiol Immunol. 1990;166:175–181. doi: 10.1007/978-3-642-75889-8_22. [DOI] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D. L., Bath M. L., Adams J. M., Cory S., Harris A. W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis D. J., Sorenson C. M., Shutter J. R., Korsmeyer S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993 Oct 22;75(2):229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]