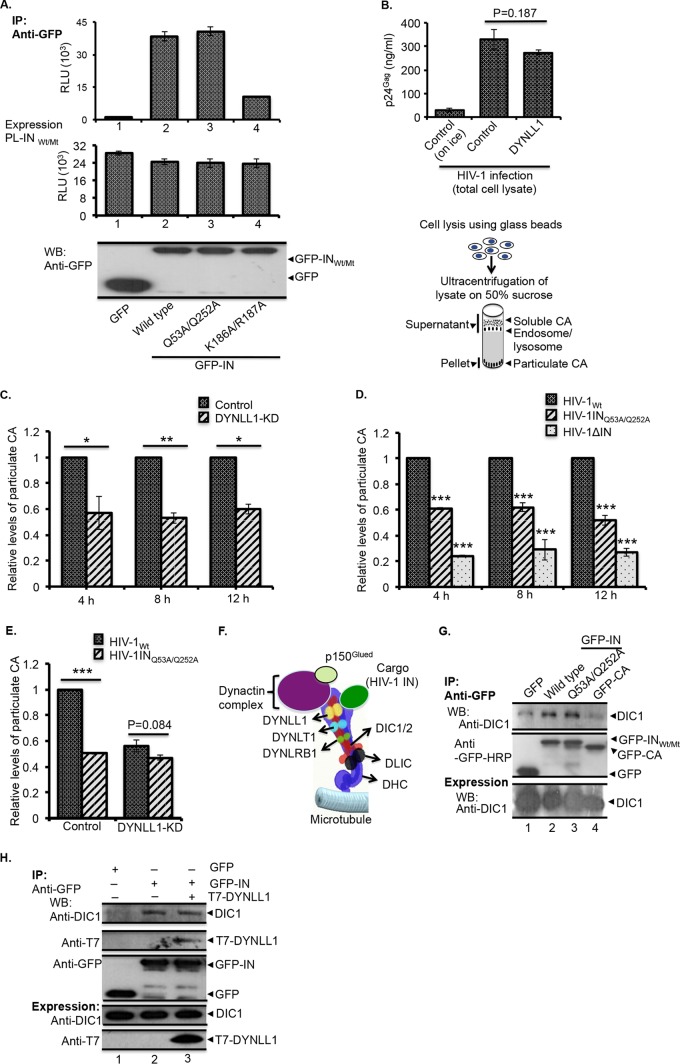
FIG 5.
HIV-1 exhibits premature uncoating in DYNLL1-KD cells or after infection with HIV-1INQ53A/Q252A mutant virus. (A) 293T cells were cotransfected with various GFP-INWt/Mt or PL-INWt/Mt fusion protein expression vectors as follows: GFP with PL-INWt (bar 1), GFP-INWt with PL-INWt (bar 2), GFP-INQ53A/Q252A with PL-INQ53A/Q252A (bar 3), or GFP-INK186A/R187A with PL-INK186A/R187A (bar 4). After 48 h of transfection, the cells were lysed and the lysates were immunoprecipitated using an anti-GFP antibody. The coprecipitation of PL-INWt/Mt in the immunoprecipitates was examined by measuring the PL activity (top panel). The expression of PL-INWt/Mt was detected by measuring the PL activity (middle panel), and the expression of the GFP-INWt/Mt protein was detected by probing the WB with the anti-GFP antibody (bottom panel). RLU, relative light units. (B) Top, representative data showing the similar levels of HIV-1 infection in control and DYNLL1-KD cells during the fate-of-capsid assay as determined by HIV-1Gag levels in total cell lysates. The statistical significance was analyzed using a Student t test. *, P = 0.187 (n = 3). Bottom, diagram showing the different steps of the fate-of-capsid assay, as described in Materials and Methods. (C) Control or DYNLL1-KD C8166T cells were infected with the HIV-1Wt virus (at 25 ng of virus-associated p24Gag/106 cells). At 4, 8, and 12 h postinfection, the HIV-1 uncoating was analyzed by the fate-of-capsid assay, as described in Materials and Methods. The data were interpreted as the fold difference in particulate CA concentrations between the control and DYNLL1-KD cell infections. The statistical significance of the differences observed between the control and DYNLL1-KD cell infections was determined using a Student t test, *, P < 0.05; **, P < 0.01 (n = 3). (D) C8166T cells were infected with equal amounts of the HIV-1Wt, HIV-1INQ53A/Q252A, or HIV-1ΔIN virus (at 25 ng of virus associated p24Gag/1 × 106 cells). At 4, 8, and 12 h p.i., the HIV-1 uncoating was analyzed by the fate-of-capsid assay. The data were interpreted as the fold differences in particulate CA concentrations between wild-type and mutant virus infections. A two-way ANOVA analysis was performed to determine the statistical significance between the samples. ***, P < 0.001 (n = 3). (E) Control and DYNLL1-KD C8166T cells were infected with the HIV-1Wt or HIV-1INQ53A/Q252A virus (at 25 ng of virus associated p24Gag/1 × 106 cells). At 4 h p.i., HIV-1 uncoating was analyzed by fate-of-capsid assay, and the data were interpreted as fold differences in particulate CA concentrations between wild-type and mutant virus-infected DYNLL1-KD or control cells. The Student t test was used to determine the statistical significance. ***, P < 0.001 (n = 3). (F) Different components of the dynein complex and the recruitment of cargo to the dynein complex through simultaneous interactions of DYNLL1 with DIC1/2 and cargo (HIV-1 IN). DYNLRB1, dynein light chain roadblock-type 1; DLIC, dynein light intermediate chain; DHC, dynein heavy chain. (G) GFP, GFP-INWt, GFP-INQ53A/Q252A, and GFP-CA fusion proteins were expressed in 293T cells, and the coprecipitation of endogenous DIC1 (top panel) was detected by Co-IP using an anti-GFP antibody. The immunoprecipitation of GFP, GFP-INWt, GFP-INQ53A/Q252A, or GFP-CA was detected by WB using an anti-GFP antibody (middle panel). DIC1 expression in the total cell lysate was detected by WB using an anti-DIC1 antibody (bottom panel). (H) GFP or GFP-IN was coexpressed with or without T7-DYNLL1 in 293T cells. The coprecipitation of DIC1 (top panel) or T7-DYNLL1 (second panel from the top) was detected by Co-IP using an anti-GFP antibody. The immunoprecipitation of GFP or GFP-IN was detected by WB (third panel from the top) using an anti-GFP antibody. The expression of T7-DYNLL1 and DIC1 in total cell lysate was detected by WB using the corresponding antibodies (bottom two panels).