Abstract
Pain remains one of the main reasons for medical consultation worldwide: moderate- to severe-intensity pain occurs in 19% of adult Europeans, seriously affecting the quality of their social and working lives. Nonsteroidal anti-inflammatory drugs (NSAIDs) are not recommended for long-term use and a careful surveillance to monitor for toxicity and efficacy is critical. This study aims to assess: 1) the pattern of use of NSAIDs and opioids in a population covered by a cloud-based pharmacovigilance surveillance system; and 2) potential inappropriate use. A retrospective 18-months systematic analysis on patients’ pain treatment was performed. The primary endpoint was evaluating the prevalence of NSAIDs and opioids use and the duration of therapy regimen. The secondary endpoint was to investigate the prevalence of NSAIDs taken for >21 consecutive days concomitant with drugs for peptic ulcer and gastroesophageal reflux disease (GORD) or antiplatelet drugs. The yearly cost for individual users of concomitant NSAIDs for more than 21 consecutive days and of GORD medications has been estimated. A total of 3,050 subjects with chronic pain were enrolled; 97% of them took NSAIDs for >21 consecutive days; about one-fourth of these users also received drugs for peptic ulcer and GORD (Anatomical Therapeutic Chemical code A02B). The yearly cost foran individual who uses NSAIDs for >21 consecutive days as well as concomitant GORD medications is 61.23 euros. In total, 238 subjects (8%) using NSAIDs for >21 days also received one antiplatelet agent. About 11% of subjects received opioids at least once and only 2% of them carried on the therapy for more than 90 consecutive days. In evaluating the escalation in dosage as a proxy of dependence risk, this study shows no dosage escalation in our cohort of chronic pain population - that is to say we show no risk of dependence.
Keywords: pain therapy, economic impact, dependence
Introduction
Pain remains one of the main reasons for medical consultation worldwide: among primary care appointments, 22% focus on pain management.1
The first large-scale computer-assisted survey undertaken to explore the prevalence, severity, treatment, and impact of chronic pain (CP) in 15 European countries and Israel concluded that CP of moderate to severe intensity occurs in 19% of adult Europeans, seriously affecting the quality of their social and working lives.2
Numerous organizations and scientific associations have made efforts to find solutions for this problem and to facilitate the treatment of pain. In 1986, the World Health Organization (WHO) presented the analgesic ladder as a framework that physicians could use when developing treatment plans for cancer pain, later extended also to nonmalignant CP.3 The WHO analgesic ladder for the treatment of CP provides a three-step sequential approach for analgesic administration based on pain severity that has global applicability. Nonopioid analgesics/nonsteroidal anti-inflammatory drugs (NSAIDs) were recommended for mild pain, with the addition of mild opioids for moderate pain and strong opioids for severe pain.
This therapeutic guideline paved the way for considerable improvements in the management of CP, and after 28 years of use, the analgesic ladder has demonstrated its effectiveness and widespread usefulness; however, modifications are necessary to ensure its continued use for knowledge transfer in pain management.4
The European Breivik screening has shown that the most common medicines taken for CP were NSAIDs (44%), followed by weak opioid analgesics (23%) and paracetamol (18%). Only 5% of patients were taking a strong opioid analgesic.2 Osteoarthritis and rheumatoid arthritis combined are the most common cause of pain in Europe (42%),2 but systematic reviews do not support long-term use of NSAIDs for these conditions.5 Also, in the USA, opioid use in older adults visiting clinics more than doubled between 1999 and 2010, and occurred across a wide range of patient characteristics and clinical settings.6 A recent African survey investigating doctors’ knowledge and attitude of treating CP demonstrated that only 9.5% of physicians use opioids for CP compared to 73% who use NSAIDs.7
NSAIDs are not recommended for long-term use, and a careful surveillance to monitor for toxicity and efficacy is critical.8 Each year in the USA, the side-effects of long-term NSAID use cause nearly 103,000 hospitalizations and 16,500 deaths. This figure is similar to the annual number of deaths from AIDS and considerably greater than the number of deaths from asthma and cervical cancer.9
The risks from chronic use of NSAIDs are significant. They can cause life-threatening ulcers and gastrointestinal bleeding, a side-effect that occurs more frequently and with greater severity as people age.10
Epidemiological studies, open-label studies, meta-analyses, and reviews concluded that this undesired effect of traditional NSAIDs also affects selective cyclooxygenase-2 (COX-2) inhibitors, called coxibs.11 Non-aspirin NSAIDs may lead to a 53% increased risk for atrial fibrillation,12 and some nonselective NSAIDs can compromise the cardio-preventive efficacy of aspirin, blocking the active site of COX-1.13 NSAIDs can worsen arterial hypertension to where is not controllable with drugs, and impair kidney function.14,15
Use of non-aspirin NSAIDs is associated with increased risk of non-Hodgkin lymphoma,16 and about one-third of people with chronic urticaria have severe reactions to NSAIDs, involving angioedema and anaphylaxis after their administration.17 NSAIDs use may mask initial pneumococcal pneumonia symptoms and delay antimicrobial therapy, thus predisposing patients to worse outcomes.18
This study aims to assess: 1) the pattern of use of pain medications, NSAIDs with Italian Agency of Medicines (AIFA) note number 66, and opioid analgesics, in a population covered by a cloud-based pharmacovigilance surveillance system; and 2) potential inappropriate use.
Materials and methods
In this retrospective study, we performed a systematic analysis of patients’ pain treatment in order to detect potential errors in pain management. Therapy errors were identified through a cloud-based pharmacovigilance surveillance system using a closed loop system that records and updates, by specifically designed software interfaces loaded on information and communication technology programs, all the drugs taken during therapy cycles.
Subjects agreeing to participate in this pharmacovigilance surveillance system were followed up for the purchases of prescription/over the counter (OTC) medications, thus generating a personal pharmacologic profile. For each subject participating, demographic data were recorded at the enrollment in the surveillance system.
Each subject was anonymously linked through an alphanumeric code to all prescription/OTC purchases in a 18-month period of: NSAIDs listed in AIFA note number 66 (AIFA note number 66 describes the active ingredient of drugs regarding which prescriptions should be restricted to a limited pathology and number of days) (Table 1); and (Figure 1) opioid drugs (Anatomical Therapeutic Chemical code [ATC] N02A). All medications were identified on the basis of the WHO ATC classification code.
Table 1.
Italian Agency of Medicines list
| International codes assigned by ATC classification system | Active ingredient |
|---|---|
| M01AB16 | aceclofenac |
| M01AB01 | indometacin |
| M01AB02 | sulindac |
| M01AB05 | diclofenac |
| M01AB51 | indometacin, combinations |
| M01AB55 | diclofenac, combinations |
| M01AC01 | piroxicam |
| M01AC02 | tenoxicam |
| M01AC06 | meloxicam |
| M01AC56 | meloxicam, combinations |
| M01AE01 | ibuprofen |
| M01AE02 | naproxen |
| M01AE03 | ketoprofen |
| M01AE09 | flurbiprofen |
| M01AE11 | tiaprofenic acid |
| M01AE51 | ibuprofen, combinations |
| M01AE52 | naproxen and esomeprazole |
| M01AE53 | ketoprofen, combinations |
| M01AH01 | celecoxib |
| M01AH05 | etoricoxib |
| M01AX17 | nimesulide |
| M01AB11 | acemetacin |
| M01AG01 | mefenamic acid |
| M01AE49 | furprofen |
| M01AE14 | dexibuprofen |
| M01AB10 | fentiazac |
| M01AX01 | nabumetone |
| M01AB14 | proglumetacin |
| M01AE12 | oxaprozin |
| M01AC05 | lornoxicam |
| M01AC01 | cinnoxicam |
Abbreviation: ATC, Anatomical Therapeutic Chemical code.
Figure 1.
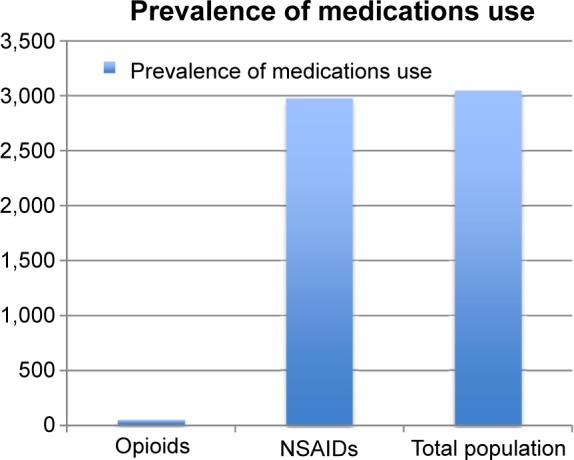
Prevalence.
Abbreviation: NSAIDs, nonsteroidal anti-inflammatory drugs.
The prevalence of use of NSAIDs and opioid medications was calculated as the ratio between the number of subjects with at least one prescription or prescription redemption/purchase of the medications of interest during the study period and the total number of subjects enrolled in the surveillance system. The duration of use was estimated on the basis of: 1) the posology recommended by the Investigator Medical Product Dossier (IMPD); 2) the number of units; 3) the number of medication packages; and 4) the number of refills. For NSAIDs, we defined appropriate use as use with a consecutive duration of ≤21 days.
The prevalence of patients who were overexposed (>21 days) to NSAIDs and who had undertaken therapy with drugs for peptic ulcer and gastroesophageal reflux disease (GORD) (ATC A02B) or antiplatelet drugs (cardioaspirin, ticlopidine, clopidogrel, or prasugrel) was analyzed, matching prescription/purchase data in a dynamic timeline. The yearly cost for individual users of concomitant NSAIDs for more than 21 consecutive days and of GORD medications was estimated. For opioids, posology escalation, defined as incremental dosage regimen, was assessed as a proxy of the risk of dependence. Statistical analysis was performed using SAS© statistical package 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 3,050 subjects with CP, 62.5% aged 39–64 years and 65% women, were enrolled. About 2,980 subjects took NSAIDs for more than 21 days consecutively in 18 months (Figure 1) (mean age 52.4 years, 43.2% women). The consecutive use of NSAIDs for more than 21 days was defined as a potential inappropriate use by AIFA.19 The three most used medications were ibuprofen (27.49%), ketoprofen (15.27%), and diclofenac (10.67%).
NSAID treatment time lasted for a mean duration of 30.43 consecutive days for women and 30.37 days for men. About one-fourth of subjects using NSAIDs for >21 days also received drugs for peptic ulcer and GORD (ATC A02B). Among medications for peptic ulcer and GORD, the most used class was proton-pump inhibitors (82%; N=570).
The yearly cost for an individual using NSAIDs for >21 consecutive days concomitant with GORD medications is 61.23 euros. Costs incurred until the 21st day are defined as appropriate and thus were not included.
In total, 238 subjects using NSAIDs for >21 days received also one of the following antiplatelet agents: acetylsalicylic acid (B01AC06), ticlopidine (B01AC05), or clopidogrel (B01AC04). Among these, the most used antiplatelet agent was acetylsalicylic acid (91%).
About 2,400 subjects, 880 men, used opioids at least once (Figure 1); 48 of them (2%) carried on with the therapy for more than 90 days consecutively. Opioid treatment lasted for a mean duration of 158 consecutive days for women and 191 days for men.
Discussion
In 2010, the Italian Ministry of Health issued the Law 38 on “Measures to ensure access to palliative care and pain therapy”.20 This is a highly innovative law which, for the first time, provides access to palliative care and pain therapy, defining the basic levels of care in order to ensure respect for dignity and health rights, equity in access to and quality of care, and their appropriateness with regard to the specific requirements. Four years after its introduction, the weak point remains the excessive and inappropriate use of NSAIDs in Italy, which have a yearly cost of over 500 million euros compared to less than 140 million euros per year for opioids.21 The current systematic analysis confirms this trend: over 97% of the cohort used NSAIDs for >21 days consecutively, and this amount is even higher than the total number of subjects who received opioids only once.
About 8% of subjects using NSAIDs for >21 days also received one of the following antiplatelet agents: acetylsalicylic acid (B01AC06, 91%), ticlopidine (B01AC05), or clopidogrel (B01AC04). Antiplatelet agents should be used with caution in patients receiving treatment with NSAIDs, including COX-2 inhibitors.22
One-fourth of subjects using NSAIDs for >21 days, also received drugs for peptic ulcer and GORD (ATC A02B). Assuming that the prevalence of concomitant NSAIDs for more than 21 consecutive days and GORD medications use of 3% found in this population is similar in the general Italian population, with a yearly cost of 108,000.00 euros.
About 2,400 subjects used opioids at least once and only 2% of them carried on with the therapy for more than 90 days consecutively. This study shows no dosage escalation in our cohort of chronic pain population - that is to say we show no risk of dependence. While no escalation in dosage was highlighted, we can confidently assume that in this cohort, long-term opioid use (>90 days) describes a proxy of tolerance and consequently dependence risk free for the period in study.
To interpret the results, it is important to highlight that the study population was composed of subjects participating in a pharmacovigilance surveillance system and not of in- or outpatients with CP.
Strengths
In this study, the availability of information on OTC purchases allowed us to profile all pain medications. Lack of information on OTC use is a limitation of several studies assessing use of pain medications, in particular NSAIDs. It is important to profile all pain medications to assess polypharmacy.23
Limitations
The study population was composed of subjects who agreed to participate in a pharmacovigilance surveillance system. These subjects may not represent the general population, eg, they may be more aware of the clinical relevance. The generalizability of results to the general population may therefore be limited.
The purchase of medication does not confirm with the actual usage of the medications, so this data could overestimate the results. However, by correlating the frequency of medication refills and prescriptions, we can assume that drugs purchased were also taken.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rasu RS, Vouthy K, Crow AN, et al. Cost of pain medication to treat adult patients with nonmalignant chronic pain in the United States. J Manag Care Pharm. 2014;20(9):921–928. doi: 10.18553/jmcp.2014.20.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Balding L. The World Health Organisation analgesic ladder: its place in modern Irish medical practice. Ir Med J. 2013;106(4):122–124. [PubMed] [Google Scholar]
- 4.Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician. 2010;56(6):514–517. e202–e205. English, French. [PMC free article] [PubMed] [Google Scholar]
- 5.Bjordal JM, Ljunggren AE, Klovning A, Slørdal L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ. 2004;329(7478):1317. doi: 10.1136/bmj.38273.626655.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman MA, Komaiko KD, Fung KZ, Ritchie CS. Use of opioids and other analgesics by older adults in the United States, 1999–2010. Pain Med. 2015;16(2):319–327. doi: 10.1111/pme.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanya EO, Kolo PM, Makusidi MA. A survey on doctors’ knowledge and attitude of treating chronic pain in three tertiary hospitals in Nigeria. Niger Med J. 2014;55(2):106–110. doi: 10.4103/0300-1652.129635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler TO, Durham CO, Planton J, Edlund BJ. Use of nonsteroidal anti-inflammatory drugs in the older adult. J Am Assoc Nurse Pract. 2014;26(8):414–423. doi: 10.1002/2327-6924.12139. [DOI] [PubMed] [Google Scholar]
- 9.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3(1):55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 10.Castellsague J, Pisa F, Rosolen V, et al. Risk of upper gastrointestinal complications in a cohort of users of nimesulide and other nonsteroidal anti-inflammatory drugs in Friuli Venezia Giulia, Italy. Pharmacoepidemiol Drug Saf. 2013;22(4):365–375. doi: 10.1002/pds.3385. [DOI] [PubMed] [Google Scholar]
- 11.Pellicano R. Gastrointestinal damage by non-steroidal anti-inflammatory drugs: updated clinical considerations. Minerva Gastroenterol Dietol. 2014;60(4):255–261. [PubMed] [Google Scholar]
- 12.Liu G, Yan YP, Zheng XX, et al. Meta-Analysis of nonsteroidal anti-inflammatory drug use and risk of atrial fibrillation. Am J Cardiol. 2014;114(10):1523–1529. doi: 10.1016/j.amjcard.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Flipo RM. Are the NSAIDs able to compromising the cardio-preventive efficacy of aspirin? Presse Med. 2006;35(Suppl 1):53–60. doi: 10.1016/S0755-4982(06)74941-7. French. [DOI] [PubMed] [Google Scholar]
- 14.Serveaux M, Burnier M, Pruijm M. Drugs: an underestimated cause of arterial hypertension. Rev Med Suisse. 2014;10(441):1661–1662. French. [PubMed] [Google Scholar]
- 15.McDowell K, Clements JN. How can NSAIDs harm cardiovascular and renal function? JAAPA. 2014;27(4):12–15. doi: 10.1097/01.JAA.0000444738.62411.83. [DOI] [PubMed] [Google Scholar]
- 16.Ye X, Casaclang N, Mahmud SM. Use of non-steroidal anti-inflammatory drugs and risk of non-Hodgkin lymphoma: a systematic review and meta-analysis. Hematol Oncol. 2014 Oct 23; doi: 10.1002/hon.2165. Epub. [DOI] [PubMed] [Google Scholar]
- 17.Drug Allergy: Diagnosis and Management of Drug Allergy in Adults, Children and Young People. National Clinical Guideline Centre (UK) 2014 Sep; [PubMed] [Google Scholar]
- 18.Messika J, Sztrymf B, Bertrand F, et al. Risks of nonsteroidal antiinflammatory drugs in undiagnosed intensive care unit pneumococcal pneumonia: younger and more severely affected patients. J Crit Care. 2014;29(5):733–738. doi: 10.1016/j.jcrc.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Mangoni AA, Knights KM. Non-steroidal anti-inflammatory drugs and risk of stroke in older patients: current controversies and research directions. G Ital Cardiol (Rome) 2011;12(5):341–349. doi: 10.1714/643.7499. [DOI] [PubMed] [Google Scholar]
- 20.Mammucari M, Muscas F, Arpino G, Aronica A, Russo P, Visconti M. Role of intensive medical training on Law 38 to improve pain management in primary care. Recenti Prog Med. 2014;105(4):159–165. doi: 10.1701/1459.16129. Italian. [DOI] [PubMed] [Google Scholar]
- 21. http://www.impactproactive.it [homepage on the Internet] Florence: Impact Proactive; [Accessed 19 March 2015]. Available from: http://www.impactproactive.it. [Google Scholar]
- 22.Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118(18):1894–1909. doi: 10.1161/CIRCULATIONAHA.108.191087. [DOI] [PubMed] [Google Scholar]
- 23.Giummarra MJ, Gibson SJ, Allen AR, Pichler AS, Arnold CA. Polypharmacy and Chronic Pain: Harm Exposure Is Not All about the Opioids. Pain Med. 2014 Oct 3; doi: 10.1111/pme.12586. Epub. [DOI] [PubMed] [Google Scholar]


