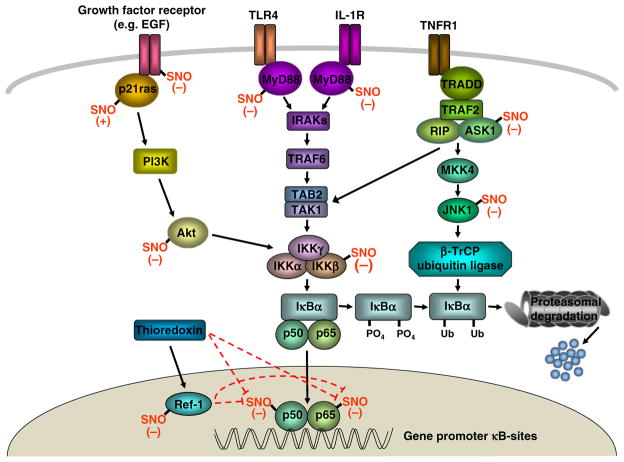
Fig. 1.
S-nitrosylation in NF-κB signaling. Engagement of cell surface receptors [TNFα (TNFR1), IL-1 (IL-1R), TLR-4, growth factor receptor] leads to the recruitment of receptor-associated proteins, several of which, p21ras, epidermal growth factor (EGF) tyrosine kinase, and MyD88, are regulated by S-nitrosylation. TNFR1 also activates the JNK kinase pathway which can enhance NF-κB signaling via the effects of the E3 ubiquitin ligase SCF-β-TrCP and IκBα proteasomal degradation. S-nitrosylation of two JNK pathway proteins, apoptosis signaling kinase 1 (ASK1) and JNK1, inhibit SCF-β-TrCP activation. Phosphorylation of IκBα, which targets it for ubiquitin-mediated proteasomal degradation, is inhibited by SNO modification of the IκBα kinaseβ (IKKβ) subunit or the ras pathway protein Akt. DNA binding of the NF-κB activating heterodimer (p50-p65) is inhibited by S-nitrosylation of either subunit. Thioredoxin can denitrosylate NF-κB p50-p65 directly or indirectly via activation of Ref-1 in the nucleus. S-nitrosylation, in turn, inhibits the activity of thioredoxin and Ref-1.