Figure 2. Role of STAT downstream of Pvr.
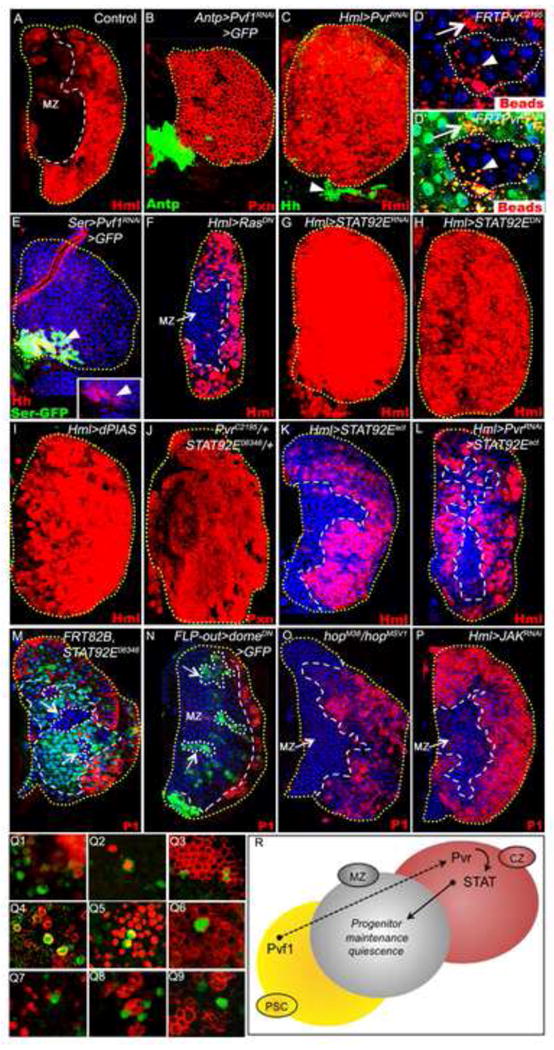
For uniformity, differentiating hemocytes are shown in red even if they are marked with EGFP (Hml-gal4 UAS-2xEGFP). Lymph glands shown are from wandering third-instar larvae. TOPRO3 marks nuclei (blue).
(A) Control. Normal Hml-gal4 expression pattern (Hml, red).
(B) Pvf1RNAi expression in the PSC (green; Antp-gal4 UAS-2xEGFP UAS-Pvf1RNAi). All non-PSC cells of the lymph gland express Peroxidasin (Pxn, red).
(C) PvrRNAi expression in the CZ (Hml-gal4 UAS-2xEGFP UAS-PvrRNAi) causes MZ progenitors to differentiate (Hml, red), although Hedgehog (green) expression in the PSC (arrowhead) remains normal.
(D and D′) Pvr-mutant clones (PvrC2195/PvrC2195, non-green cells) differentiate normally as judged by their ability to phagocytose FluoSphere beads (red), similar to neighboring wild type tissue (green).
(E) Hedgehog expression (red, inset) is unaffected when Pvf1RNAi is expressed in the PSC (green; Ser-gal4 UAS-2xEGFP UAS-Pvf1RNAi); yellow represents co-localization of Hedgehog and Ser-gal4 expression.
(F) Expression of RasDN in differentiating hemocytes (Hml-gal4 UAS-2xEGFP UAS-RasDN) does not affect progenitor fate.
(G-I) Loss of STAT function in differentiating cells causes progenitor differentiation (Hml, red). This phenotype can be induced by expressing either (G) Stat92ERNAi (Hml-gal4 UAS-2xEGFP UAS-Dcr-2 UAS-Stat92ERNAi), (H) Stat92EDN (Hml-gal4 UAS-2xEGFP UAS-Stat92EDN), or (I) dPIAS (Hml-gal4 UAS-2xEGFP UAS-dPIAS).
(J) Combined single-copy-loss of Pvr and Stat92E (PvrC2195/+; Stat92E06346/+). All progenitors express Pxn (red).
(K) As a control, expression of Stat92Eact (Ekas et al., 2010) in differentiating cells has no effect on progenitor fate (Hml-gal4 UAS-2xEGFP UAS-Stat92ΔNΔC) (L) Co-expression of Stat92Eact and PvrRNAi (Hml-gal4 UAS-2xEGFP UAS-Stat92Er UAS-PvrRNAi) suppresses the progenitor differentiation phenotype caused by PvrRNAi alone (compare with C).
(M) Stat92E mutant clones (Stat92E06346/Stat92E06346) within the MZ (lacking green, demarcated by white dots) do not cause ectopic differentiation (lack of red within the clones).
(N) Blocking Domeless function in MZ cells (FLP-out gal4 UAS-domeDN clones, green, see methods for details, demarcated by white dots), does not cause them to differentiate (P1, red).
(O) JAK mutant animals (hopM38/hopMSV1) do not exhibit ectopic differentiation (P1, red) of progenitors.
(P) Expression of JAKRNAi specifically in differentiating cells (Hml-gal4 UAS-2xEGFP UAS-hopRNAi) does not cause MZ progenitor differentiation (P1, red).
(Q1-9) In the third instar, lymph glands exhibit a small fraction of cells that express a reporter of STAT activity (green, 10X Stat92E-GFP). These cells are negative for domeless (dome-MESO-lacZ; Q1), proliferate (PH3; Q2), and express Pvract (Q3-4) and Hml (Hml-ga4, UAS-lacZ; Q5), but lack differentiation markers: Pxn (Q6), Lz (Q7), ProPO (Q8) and P1 (Q9). Cells appear yellow due to co-localization of the STAT reporter and Hml (anti-|3-gal), Pvract, or PH3.
(R) Schematic representation of STAT function in progenitor maintenance. Pvf1 from the PSC is transported to the differentiating cells in the CZ to activate its receptor Pvr, leading to the activation of STAT that, in turn, generates the CZ signal necessary for the maintenance of the progenitors in the MZ. Thus, loss of either Pvf1 from the PSC (in B), or loss of Pvr (in C) or STAT (in G-I) from the CZ results in proliferation and differentiation of MZ progenitors.
