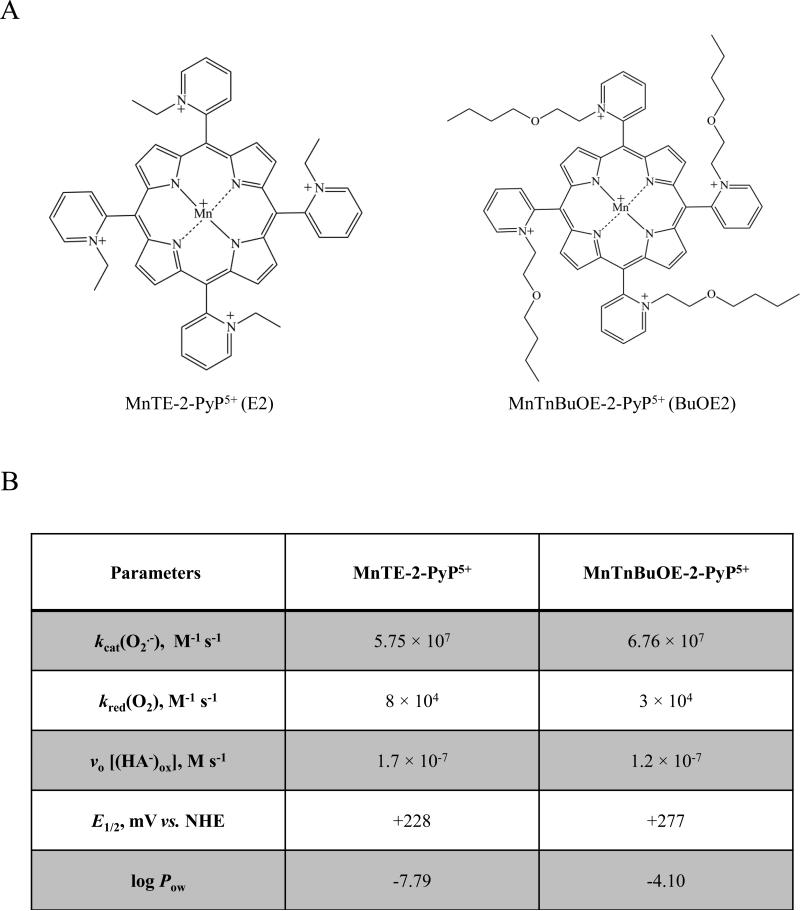
Figure 1. Structure, physicochemical characteristics, and redox properties of two Mn porphyrins used in this study.
A) Chemical structures for the two manganese porphyrins MnTE-2-PyP5+ (E2) and MnTnBuOE-2-PyP5+ (BuOE2) used in this study. B) Table contains the catalytic rate constant for O2•- dismutation, kcat(O2•-), which describes their SOD-like activities; the rate constant for one-electron reduction of molecular oxygen, O2, to superoxide, O2•-, kred(O2); the initial rate of ascorbate oxidation, vo [(HA−)ox]; the metal-centered reduction potential for MnIIIP/MnIIP redox couple, E1/2 in mV vs. NHE; and the partition coefficient for n-octanol and water (log Pow,) as a measure of lipophilicity. The values presented were compiled from [24, 33]. Two kinetic approaches were used to evaluate the efficacy of MnPs as catalysts in the ascorbate-oxidation driven H2O2 production: Marcus equation and initial rates. aThe kred(O2) was estimated based on Marcus equation as previously reported [46]. bThe initial rates of ascorbate oxidation by MnPs, were determined spectrophotometrically following the decrease in the ascorbate absorption at 265 nm over 5 minutes.