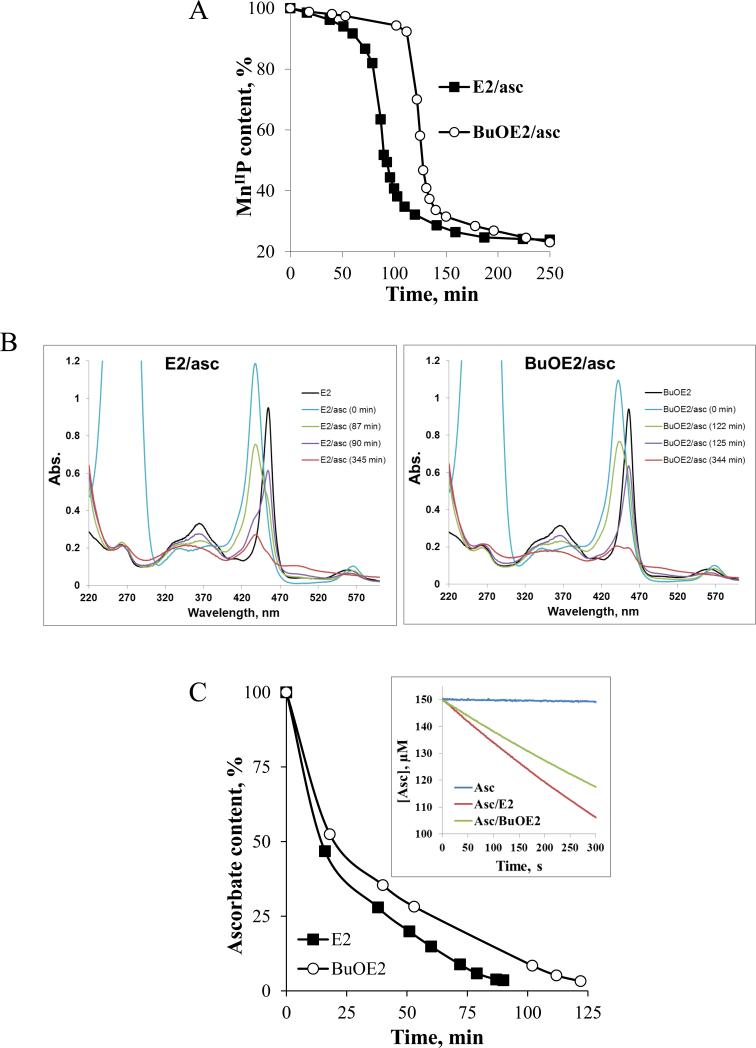
Figure 7. H2O2-driven degradation of Mn porphyrins in the presence of ascorbate, and oxidation/consumption of ascorbate.
A) Ascorbate (0.42 mM)-driven reduction of MnIIIP (6 μM) and its subsequent H2O2-driven oxidative degradation , as registered spectrophotometrically at the Soret band of the reduced MnIIP (437.5 nm for E2 and 441.5 nm for BuOE2) ; as Mn oxo species appear over time, our data do not allow for the assessment of the total MnP content. As long as ascorbate was present in solution it kept reduced MnIIP in solution (indicated as flat line); once ascorbate is consumed and peroxide accumulated, MnIIP gets reoxidized to MnIIIP which subsequently reacts with H2O2. The oxidation of MnP with H2O2 leads to its degradation and is indicated with a large drop in MnP absorption (see below); B) and C) Time dependent spectral change of MnPs in the presence of ascorbate. The first spectrum was recorded in the absence of ascorbate (HA−), described as E2 or BuOE2, and relates to MnIIIP. Upon addition of ascorbate, the MnIIIP got reduced to MnIIP (E2/asc, 0 min and BuOE2/asc, 0 min) which stays present in solution as long as there is ascorbate available. Once the ascorbate is consumed (disappearance of band at 265 nm), under aerobic conditions, the MnP ceased to be maintained in a reduced form. Consequently, the disappearance of the reduced MnIIP was observed, which was accompanied with the reappearance of MnIIIP (~90 min for E2 and 125 min for BuOE2). The MnIIIP subsequently got oxidized with H2O2 to a highly oxidizing Mn(V) oxo species, (O)2MnVP (eq [5]), which in turn oxidizes the porphyrin ring thus leading to its degradation. The (O)2MnVP readily decays to O=MnIVP [48, 49]. The Soret bands of differently oxidized/reduced E2 species, that are present in solution at time-dependent ratios, are: MnIIP at 438 nm, ε = 1.81 × 105 M−1 s−1, MnIIIP at 454 nm, ε = 1.29 × 105 M−1 s−1, O=MnIVP at 425 nm, ε = 9.0 × 104 M−1 s−1, and (O)2MnVP (pH 14) at 433 nm, ε = 1.38 × 105 M−1 cm−1 [49]. C) Time-dependent oxidation/consumption of ascorbate, based on the spectrophotometric measurements of ascorbate at 265 nm, is accompanied by peroxide accumulation in the system. Inset of Figure 7C: Initial rates of ascorbate oxidation catalyzed by two Mn porphyrins differ by ~30%. The conditions are: 5 μM MnP, 150 μM ascorbate in 50 mM Tris buffer (pH 7.8). Initial rates are: for noncatalyzed HA− oxidation, vo(HA−)ox = 3.0 × 10−9 M s−1; for E2-catalyzed HA− oxidation, vo(HA−)ox = 1.7 × 10−7 M s−1; and for BuOE2-catalyzed HA− oxidation vo(HA−)ox = 1.2 × 10−7 M s−1 (Figure 1B). All experiments were performed in a cell-free tris-buffered system (pH 7.8).