Figure 2.
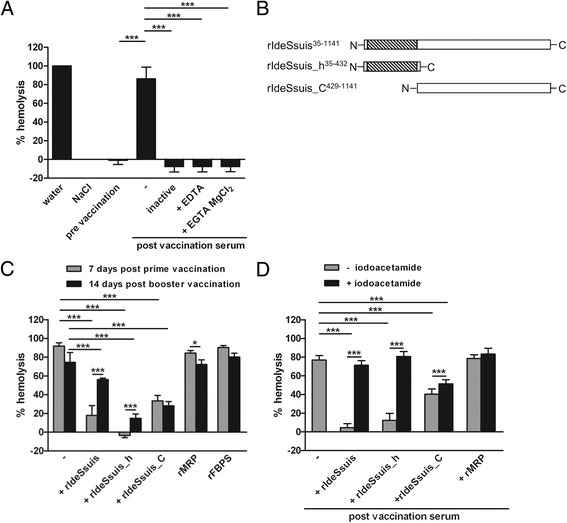
Hemolysis caused by the classical complement activation pathway is abrogated by Ide Ssuis in dependence of the protease activity. (A) Purified sheep erythrocytes were incubated with water (defined as 100% hemolysis), physiological sodium chloride solution (NaCl), sera of a piglet drawn before (pre vaccination serum) and 7 days after prime vaccination (post vaccination αEry serum) with ovine erythrocytes. The αEry serum was heat inactivated or treated with EDTA to access the impact of complement activation. To specifically inhibit the classical complement pathway 10 mM EGTA plus 15 mM MgCl2 was used. (B) Illustration of rIdeSsuis and its truncated derivatives. The amino acids of IdeSsuis included in these constructs are superscribed. The region homologous to IdeS is shaded. (C) Complement dependent hemolysis is reduced by pretreatment of the indicated different αEry sera with rIdeSsuis, rIdeSsuis_homologue (rIdeSsuis_h) and rIdeSsuis_C_terminus (rIdeSsuis_C) but not with rMRP and rFBPS. (D) Proteolytic activity of rIdeSsuis and rIdeSsuis_homologue is crucial for complement inhibition. The IdeSsuis constructs were incubated with the cysteine-protease inhibitor iodoacetamide prior to incubation with the αEry sera as indicated. The final dilutions of porcine sera in the hemolysis assay were 1:20 in all cases. Bars and error bars represent mean values and standard deviations, respectively. Significant differences are indicated (* p < 0.05; ** p < 0.01; *** p < 0.001).
