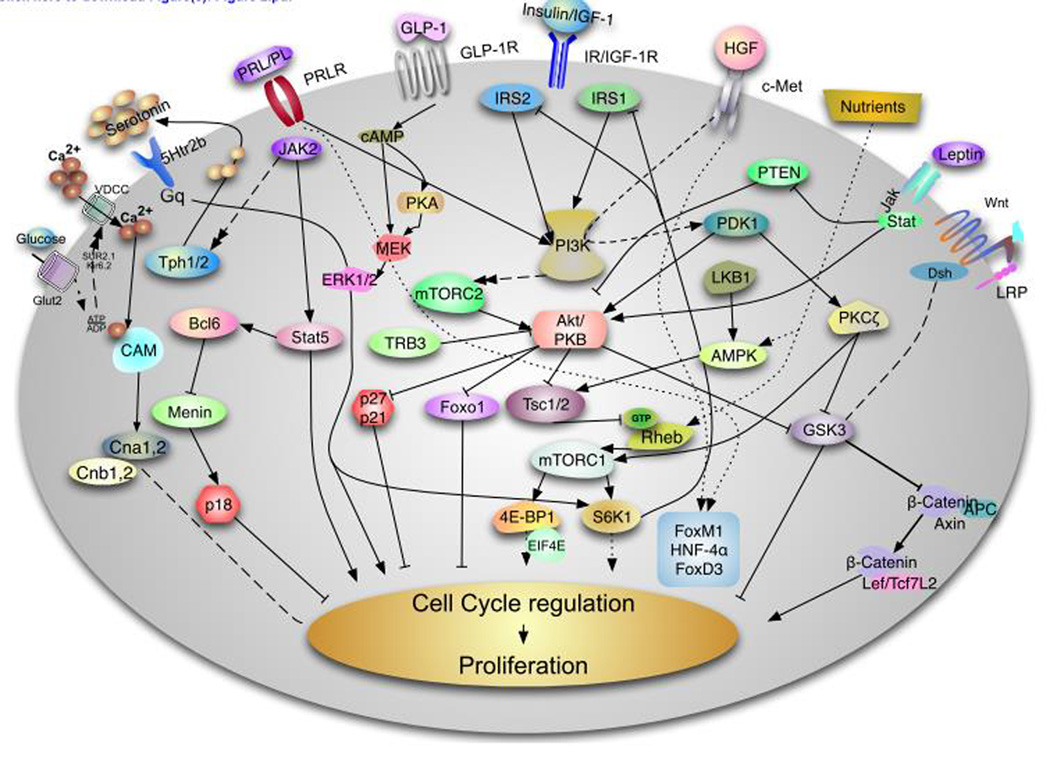
Figure 2. Signaling pathways involved in Rodent β-cell proliferation.
This diagram describes some of the major pathways responsible for rodent β-cell proliferation. This field is evolving and we have attempted to provide a summary of the major pathways involve this process. The insulin/insulin-like growth factor I (IGF1) receptor, hepatocyte growth factor and glucagon like peptide 1 (GLP1) signal through the IRS/PI3 kinase pathway to regulate Akt by modulating PDK1. Complete activation of Akt requires the phosphorylation of this kinase by mTORC2. The serine threonine kinase has been demonstrate to play a major role in regulation of β-cell proliferation, survival and cell size by acting on multiple downstream targets including FoxO1, tuberous sclerosis complex (TSC) 1 and 2, Glycogen synthase kinase (GSK) 3 and the cell cycle inhibitors p27cip and p21 among others. Akt activity is negatively regulated directly and indirectly by TRB3 and PTEN. Downstream of Akt, the TSC/mTORC1 integrates signals from growth factors and nutrients. Nutrients can modulate this pathway directly by acting on the protein Rheb or indirectly by regulating the activity of the AMPK pathway, which is also regulated by LKB1. mTORC1 regulates protein translation and cell size by regulating S6K and 4E-BPs. HGF binding to its tyrosine kinase receptor, c-Met, results PKCζ activation which in turns inactivates GSK3β and increases phosphorylation and activation of mTORC1. Activation of the prolactin receptor induces Jak2/Stat5 signaling and/or Jak2/Bcl6/Menin to induce β-cell proliferation. Recent data shows that lactogens induce proliferation by activation of tryptophan hydroxylases (Tph1/2 with generation of serotonin. Serotonin is secreted and act in paracrine fashion to assumed to act via the HTR2b receptor to modulate intracellular calcium and PKC or PI3K members. Glucose is transported by the glucose transporter Glut2 and metabolizes to increase the ATP ratio, which subsequently inhibits the Kir6.2 channel resulting depolarization and calcium influx. Intracellular calcium signaling is a major driver of β-cell proliferation by regulating multiple signaling pathways including Calmodulin (Cam) Calcineurin (Cna1,2 and Cnb1,2) and NFAT transcription. In parallel, glucose activates ChREBP and cMyc to induce cell cycle progression (not shown). Upon binding to GLP-1 GLP-1 receptor results in elevation of cAMP, protein kinase A (PKA) activation and this ultimately results in which can phosphorylation of β-catenin by MEK/ERK1/2 pathway. Leptin acts via the JAK-STAT pathway induces Akt signaling by inhibiting PTEN. The Wnt/frizzled pathway acts by inhibiting GSK-3β and blocking phosphorylation of β-catenin modulate transcription of Lef/Tcf7L2 dependent genes such as cyclin D2 and potentially cyclin D1 and cMyc and activates cell cycle progression. It is important to note that our understanding of human β-cell proliferation is notably limited compared to that of rodents.