Abstract
TOL101 is a murine IgM mAb targeting the αβ TCR. Unlike other T cell targets, the αβ TCR has no known intracellular signaling domains and may provide a nonmitogenic target for T cell inactivation. We report the 6-month Phase 2 trial data testing TOL101 in kidney transplantation. The study was designed to identify a dose that resulted in significant CD3 T cell modulation (<25 T cell/mm3), to examine the safety and tolerability of TOL101 and to obtain preliminary efficacy information. Thirty-six patients were enrolled and given 5–10 daily doses of TOL101; 33 patients completed dosing, while three discontinued after two doses due to a self-limiting urticarial rash. Infusion adjustments, antihistamines, steroids and dose escalation of TOL101 reduced the incidence of the rash. Doses of TOL101 above 28mg resulted in prolonged CD3 modulation, with rapid recovery observed 7 days after therapy cessation. There were no cases of patient or graft loss. Few significant adverse events were reported, with one nosocomial pneumonia. There were five biopsy-confirmed acute cellular rejections (13.9%); however, no donor-specific antibodies were detected. Overall TOL101 was well-tolerated, supporting continued clinical development using the dose escalating 21– 28–42–42–42mg regimen.
Keywords: Acute rejection, induction, kidney transplantation
Introduction
Despite dramatic advances in clinical immunosuppression, a persistent goal in kidney transplantation remains the control of alloimmunity while minimizing the toxicities of immunosuppressive agents. Prophylaxis against T cell–mediated (cellular) acute rejection involves inhibition of alloreactive αβ T cells immediately following or within the first few weeks after engraftment (1–3). Contemporary maintenance immunosuppressive agents such as calcineurin inhibitors (cyclosporine, tacrolimus), mammalian target of rapamycin inhibitors (sirolimus, everolimus), anti-proliferative agents azathioprine, mycophenolate mofetil (MMF) or mycophenolic acid and agents capable of altering costimulation pathways (humanized IgG/CTLA4 fusion protein; belatacept) have all combined to provide effective tools to prevent rejection (4–6). In addition, the use of antibody induction therapy not only appears to decrease the severity and rate of acute rejection but also permits a delayed introduction and minimization of potentially nephrotoxic maintenance therapy and may improve outcomes (7–9). Prophylactic (induction) antibody agents include rabbit or equine anti-thymocyte globulin (ATG), anti-IL-2 receptor antibody (basiliximab), anti-CD52 (alemtuzumab) and the recently discontinued murine anti-CD3 antibody OKT3 (10,11).
While the use of induction agents has significantly increased over the past decade, questions remain regarding the efficacy of anti-IL-2 receptor antibody and the risk/benefit balance of depletional agents. In patients with higher immunological risk, depletional therapies including ATG and alemtuzumab are typically utilized (11–13). These agents lead to prolonged mononuclear cell depletion, which is desirable under conditions of increased risk of rejection but may lead to untoward effects such as enhanced susceptibility to infection as well as the development of malignancy. They are also occasionally associated with a number of drug-related adverse events (AE) such as infusion reactions, and a first dose cytokine release syndrome characterized by IL-6 and tumor necrosis factor (TNF) release (14). Utilization is also limited in some cases in the acute setting by the development of thrombocytopenia, and in the chronic setting by the development of anti-drug antibodies (15). While the nondepleting anti-IL-2 receptor antibody basiliximab is associated with reduced side effects, its efficacy is not well defined under modern immunesuppression and is notably inferior to depletional agents when immune suppression minimization or steroid avoidance is attempted (13,16). Therefore, its use is usually restricted to patients at low risk for transplant rejection. Given the diverse spectrum of immunological risk profiles among kidney transplant recipients, there exists a therapeutic gap for an induction agent that rapidly and reliably modulates the T cell response but avoids long-term lymphodepletion.
Targeting the αβ TCR has been shown to be highly efficacious in animal models of transplantation and autoimmunity (17–20). Additionally, outcomes with predecessor anti-αβ agents in human studies not only suggest a reduced AE profile but also displayed similar efficacy to muromonab-CD3 (21–23). TOL101 is a murine IgM mAb to the αβ TCR under study as a T cell–modulating agent. Unlike predecessor anti-TCR agents including muromonab-CD3 and humanized monoclonal anti-CD3 IgG agents that targeted the CD3 subunit of the TCR, the novel targeting of TOL101 to the ab subunits of TCR has the potential to modulate T cells without mitogenicity, since the ab TCR has no known intracellular signaling domains.
In order to determine the safety and immunomodulatory effects of TOL101 as well as gather preliminary data regarding the efficacy of TOL101 in the prevention of acute T cell–mediated rejection, a first-in-human Phase 2 study of TOL101 as an induction agent for primary kidney transplant recipients was performed in 36 subjects, reported herein.
Methods
TOL101 development
TOL101 is an optimized version of the murine anti-human αβ TCR antibody originally named T10B9. This next-generation anti-αβ TCR antibody has two nucleotide mutations and a different glycosylation pattern to T10B9. In addition, the manufacturing process has been optimized, incorporating serum and animal-free components. TOL101 has been assigned a new IND #104,594.
Study design
This Phase 2 study sponsored by Tolera Therapeutics, Inc. (Kalamazoo, MI) was an open label, multicenter, first-in-human study designed to investigate the safety, preliminary efficacy and immunogenicity of TOL101 administered to primary kidney transplant recipients. The study was designed with a modified adaptive design, including an initial dose-escalation component. Subjects were enrolled from November 2010 until December 2012, ClinicalTrials.gov identifier NCT01154387. De novo renal transplant recipients (n = 36) were enrolled into successively higher dose levels with the goal of identifying two potentially therapeutic dose levels to be evaluated further in Phase 3. The initial dose levels started a one-tenth (0.28 mg) of the minimum anticipated biologic effect level (MABEL) of 2.8 mg. The follow-up period was 6 months for all 36 enrolled patients. Good Clinical Practice and Declaration of Helsinki ethical principles were followed throughout the study. Institutional Review Board approval was obtained at all participating centers, with patients receiving written informed consent upon enrollment. The sponsor, Tolera Therapeutics, monitored safety, with an independent data safety monitoring board (DSMB) used to provide safety oversight and to provide guidance on each cohort for dose escalation. Serious events and opportunistic infections were both investigator reported and confirmed by an independent monitor that reviewed the medical records of each enrolled patient.
Eligibility criteria
Eligible patients were aged 18–60 years and scheduled to receive a primary non-HLA identical kidney transplant from a living donor or standard criteria deceased donor, with cold ischemia time of <30 h. In the last cohort, two expanded criteria deceased donor (ECD) kidneys were enrolled. Only patients with a history of Epstein-Barr virus (EBV) exposure (positive IgG serology) and a panel reactive antibody of <20% were included in the study. Patients were excluded from participation if they had prior organ transplantation, positive flow cytometry T cell cross-match, an ABO-incompatible donor, white cell count <2000/mm3, platelet count <100 000/mm3, absolute neutrophil count <1000/mm3, liver transaminases three times the upper normal value, HIV infection, hepatitis B or C virus infection. X-ray confirmed chest inflammation; co-morbid conditions thought to represent excessive risk or known hypersensitivity to rodent proteins or other protocol required medications.
Maintenance immune suppression
Maintenance immunosuppression consisted of MMF, tacrolimus and tapering steroids. Oral or intravenous (IV) MMF (minimum 750mg twice daily) was initiated on the day of transplant. Tacrolimus was initiated orally between Study Day 1 and Day 6, at the discretion of the investigator. The starting dose of tacrolimus was 0.1-0.2 mg/kg/day in divided doses. Subsequent doses of tacrolimus were individualized to maintain whole blood C0 levels in the range of 6-15 ng/mL for the first month posttransplant. Tacrolimus C0 level measurements were done daily during TOL101 administration, weekly, Month 1 and on Days 90 and 180. The initial corticosteroid dose was 500 mg IV methylprednisolone at transplantation; 250 mg on Day 1; 125mg on Day 2; 60 mg Day 3; then oral prednisone 0.5 mg/kg from Day 4; tapered to 5–10 mg/day by Month 1 and to ≥5mg/day at Day 45 until Month 6.
Anti-infective prophylaxis
Oral valganciclovir (Valcyte®); Genentech, So. San Francisco, CA was recommended at starting dose of 450 mg within first 3 days of transplant in cytomegalovirus (CMV)+ recipients or in recipients of kidneys from CMV+ donor, to be given daily for 6 months in those D+R−, and 3 months in the others. Oral trimethoprim/sulfamethoxazole (Septra SS®/Bactrim®) was required for 6 months for prophylaxis of Pneumocystis carinii pneumonia, and fungal prophylaxis per institution standard of care.
TOL101 administration and pharmacodynamics
TOL101 was provided in 14mg lyophilized vials (Tolera Therapeutics, Inc.). Subjects received at least six daily doses of TOL101, beginning in the operating room on Day 0 through a central venous catheter. Initial infusions were administered over 1–2 h, and all subjects received 50 mg IV diphenhydramine and their daily methylprednisolone dose within 1 h of the first three TOL101 doses. Using CD3T cell count as the primary marker of efficacy, this study was designed to test ascending doses of TOL101. Due to the potential immune stimulatory capacity of TCR targeting antibodies, the initial TOL101 dose used was one-tenth of the MABEL, which was determined to be 0.28 mg. The precise dosing regimens in successive cohorts were Group 1: 0.28 mg (n = 2); Group 2: 2.4 mg (n = 2); Group 3: 7 mg (n = 2); Group 4: 14 mg (n = 2); Group 5: 28 mg (n = 6); Group 6: 32 mg (n = 4); Group 7: 42 mg (n = 4) and two dose-escalation cohorts. The first dose-escalating cohort Group 8: (n = 6) tested 14 mg at Day 0, 21 mg at Day 1, 28 mg at Day 2, 42 mg at Day 3 and 42 mg at Day 4, with 42 mg administered daily until target tacrolimus levels were achieved. The second dose-escalating cohort Group 9: (n = 8) tested 21 mg at Day 0, 28 mg at Day 1, 42 mg at Day 2 and 42 mg at Day 3 and 42 mg until target tacrolimus levels were achieved. A 24-h hold between patients and regular DSMB review of patient data were performed as further safety precautions. A dose was considered to be efficacious if CD3 counts were <25 T cells/mm3 throughout the dosing interval. Cessation of TOL101 was determined after a minimum of six doses, if the tacrolimus C0 levels were therapeutic (8–15ng/mL).
End points
Safety parameters
Multiple safety parameters were monitored. Events were classified by organ system as AE or serious adverse events (SAE) according to the Medra dictionary using Good Clinical Practice guidelines (http://www.who.int/medical_devices/innovation/MedDRAintroguide_version14_0_March2011.pdf). Subjects who suffered the same event more than once were recorded as suffering one event. Subjects who had more than one AE within a system organ class were counted only once in that system organ class. Immune safety parameters including symptoms that may suggest cytokine release syndrome were carefully monitored. Cytokine release syndrome was identified using a constellation of three observations after infusion, namely a fever greater than 101° Fahrenheit, combined with rigor (shaking chills) and shortness of breath. Serum levels of TNF, interferon-γ (IFN-γ), IL-2, IL-6 and IL-10 were determined at 0, 2, 8 and 24 h after the first dose, as well as upon recognition of an infusion reaction involving fever, chills, rigors, skin disorders or shortness of breath. Cytokines were measured by PRL laboratories (Overland Park, KS) using Luminex technology (Austin, TX). Nitric oxide (NO) levels were also determined using calorimetric assay by ABC Laboratories (Columbia, MI) at 0, 2, 8 and 24 h after the first dose as well as on Day 4. Human anti-mouse antibody (HAMA) was determined at baseline, Day 14 and Day 28, using sandwich enzyme-linked immunosorbent assay (ELISA) with TOL101 as the capture antibody (ABC Laboratories).
The incidence of malignancies including lymphoproliferative disorder was collected from clinical records. Screening for CMV (Days 28, 90 and 180), BK virus (BKV) (Days 90 and 180) and EBV (Days28, 90 and 180) was performed using blood polymerase chain reaction detection. The incidence of other serious or opportunistic infections was also recorded.
Efficacy parameters
Clinical efficacy was determined by the pharmacodynamic (PD) effect of TOL101 on CD3+ T lymphocyte counts. Successful T cell modulation was considered present in patients with sustained daily CD3+ T cell numbers below 25 CD3+ counts/mm3 for the continuous dosing interval. In addition, the triple end point of patient survival, graft survival and biopsy-confirmed acute rejection (BCAR) at any time during the 6 months was determined. Protocol biopsies were not required. Delayed graft function was defined as the need for dialysis within the first week posttransplant. Renal function was determined by estimated GFR (eGFR) at each study visit (MDRD method), with measured GFR determined by iothalamate clearance at Day 180/end of study (EOS). The spot urine protein to creatinine ratio as well as donor-specific antibody (DSA) was measured at Day 90 and Day 180/EOS.
CD3 counts
Flow cytometry was used to determine CD3 counts and the impact of TOL101 on memory and naïve T cell subsets. Blood samples were collected daily in the morning within 1–2h of the next TOL101 dose in sodium heparin tubes and shipped overnight to a central flow cytometry facility (Neo-Genomics Laboratories, Irvine, CA), with CD3 counts determined using the Beckman Coulter (Brea, CA) CD3 flow cytometry kit on whole blood samples.
Pharmacokinetics
Serum concentrations of TOL101 were measured at 0, 2, 4, 8 and 24h after administration of Dose 1 (Day 0), Dose 4, the last dose, and on Day 14. An ELISA for murine mouse IgM was utilized (ABC Laboratories). A one-compartment IV infusion model was used and fit using Phoenix WinNonlin Version 6.2 (Certara, St. Louis, MO) and data were weighted by 1/Yhat, where Yhat is the predicted plasma concentration at each time.
Statistics
The number of subjects per cohort was not based on statistical considerations but intended to provide safety and PD data sufficient to escalate to the next dose level. Frequency tables have been presented for all infections, AEs, all AEs by maximum severity, drug-related AEs, SAEs and AEs resulting in study drug discontinuation. For quantitative laboratory tests, summary statistics are presented at each time point. Both measured and eGFR are summarized with descriptive statistics. Delayed graft function and episodes of BCAR are summarized in frequency tables. For the urine protein to creatinine ratio and the DSA assessments, summary statistics will be presented for the values obtained at Day 90 and Day 180/EOS.
Results
Patient characteristics
Patient enrollment began in February 2010 and ended in December 2012 with 13 US centers participating. A total of 36 patients were enrolled into this Phase 2 study, including a broad cross-section of patients (Table 1). The mean donor age was 40 years, with 28 living and 8 deceased donors. The mean recipient age was 44 years, with 75% male. The final dose-escalation cohorts included eight deceased donor transplants, two ECD donor kidneys and four African American recipients, with HLA mismatch being on average greater than 4.The most common causes of end-stage renal disease were glomerular diseases (38%) and polycystic kidney disease (25%).
Table 1.
Demographics (N = 36)
| Recipients | Donors | |
|---|---|---|
| Age at transplant, mean (years) | 44.4 | 40.5 |
| Height (cm) | 177.2 | N/A |
| Weight (kg) | 91.58 | N/A |
| BMI (kg/m2) | 29.47 | N/A |
| Gender, n (%) | ||
| Male | 27 (75) | 15 (41.7) |
| Female | 9 (25) | 21 (58.3) |
| Ethnic origin, n (%) | ||
| White | 27 (75) | 21 (75) |
| Black | 6 (16) | 5 (18) |
| Asian | 1 (2.8) | 1 (3.5) |
| Other | 1 (2.8) | 1 (3.5) |
| Cause of renal failure, n (%) | ||
| Hypertensive nephrosclerosis | 5 (13.9) | N/A |
| Diabetes | 5 (13.9) | N/A |
| Polycystic kidney disease | 9 (25) | N/A |
| Glomerulonephritis | 1 (2.8) | N/A |
| Focal segmental glomerulonephritis | 3 (10.7) | N/A |
| IgA nephropathy | 5 (17.9) | N/A |
| Other | 8 (22.2) | N/A |
| Type of donor, n (%) | ||
| Living, related | N/A | 13 (46.4) |
| Living, unrelated | N/A | 15 (41.6) |
| Deceased | N/A | 8 (22.2) |
| Blood type, n (%) | ||
| A | 15 (41.7) | 11 (30.5) |
| B | 3 (8.3) | 4 (11.1) |
| AB | 3 (8.3) | 1 (2.7) |
| O | 15 (41.7) | 20 (55.5) |
| HLA mismatch, n (%) | ||
| 0 | 0 (0) | N/A |
| 1 | 2 (5.5) | N/A |
| 2 | 2 (5.5) | N/A |
| ≥3 | 32 (88.9) | N/A |
| Panel-reactive antibody at baseline | ||
| Mean (%) | 3.4 | N/A |
| ≥20, n (%) | 1 (2.8) | N/A |
| Cold ischemia time | ||
| Mean (min) | 330 | N/A |
| Pretransplant CMV antibody match, n (%) | ||
| Donor+/recipient− | 8 (22.2) | N/A |
| Donor+/recipient+ | 10 (27.8) | N/A |
| Donor−/recipient− | 13 (36.1) | N/A |
| Donor−/recipient+ | 5 (13.9) | N/A |
CMV, cytomegalovirus.
Serious adverse events
For the entire study, 27 SAEs were reported in 12 subjects (Table S1). No deaths were observed. All but one SAE was considered to be “unrelated” to study drug. The “potentially related” SAE was nosocomial pneumonia on POD 18 in a 42-year-old male. He was hospitalized with a cough and persistent chest X-ray infiltrate, but no organisms were cultured from blood or sputum. He was given antibiotics and the pneumonia resolved in 2 weeks. Otherwise, most other SAEs were commonly associated with transplant surgery and other non-TOL101-related issues.
Adverse events
For the entire study, a total of 653 AEs were reported in 36 subjects including 47 AEs in 22 subjects who were reported to be “possibly,” “probably” or “definitely” related to TOL101. All reported AEs regardless of causality observed in ≥10% of patients are shown in Table 2. The majority of AEs were reported in 28, 32, and 42 mg dose cohorts. Three subjects discontinued drug due to a drug-related AE (rash), all in the 42 mg dose group. The most commonly reported study drug-related AE observed in 11 (30%) of patients was a skin rash, which was variably described as urticarial, red, raised, hives and/or wheal like. The rash appeared on the trunk, abdomen and/or the extremities (Figure 1) and usually began at the end of the first or second infusion of TOL101 or a few hours thereafter (Tables 2 and 3). The rash resolved in all cases within a few hours spontaneously, or by the next day after treatment with additional diphenhydramine and acetaminophen. In no case was the rash necrotic or persistent, and it never progressed to more severe manifestations. The rash was most intense and only recurred in the initial 42 mg cohort. This led to the protocol of stepwise increases in the initial doses of TOL101 (the dose-escalating cohorts) and a prolongation of the infusion time to 6 h for the first two doses. This approach allowed for 42 mg dosing with reduced rash incidence. In the final cohort dose regimen of Day 0 (21 mg), Day 1 (28 mg), Day 2 (42 mg), Day 3 (42 mg) and Day 4 (42 mg), there was one transient rash, which occurred with inadvertent rapid infusion of the first dose (Table 3).
Table 2.
Adverse events (all causality) >10% in any treatment group, N = 36
| Adverse event | Patients, n (%) |
|---|---|
| All | 36 (100) |
| Blood and lymphatic disorders | |
| Total | 14 (38.9) |
| Anemia | 5 (13.9) |
| Leukopenia | 6 (16.7) |
| Cardiac disorders | |
| Total | 9 (25) |
| Tachycardia | 6 (16.7) |
| Eye disorders | |
| Total | 4 (11.1) |
| Vision blurred | 4 (11.1) |
| Gastrointestinal disorders | |
| Total | 31 (86.1) |
| Abdominal distention | 4 (11.1) |
| Abdominal pain | 5 (13.9) |
| Constipation | 16 (44.4) |
| Diarrhea | 16 (44.4) |
| Nausea | 20 (55.6) |
| Vomiting | 10 (27.8) |
| General disorders and administration site disorders | |
| Total | 24 (66.7) |
| Chills | 4 (11.1) |
| Fatigue | 10 (27.8) |
| Edema peripheral | 9 (25) |
| Pain | 4 (11.1) |
| Injury, poisoning and procedural complications | |
| Total | 33 (91.7) |
| Incision site pain | 26 (72.2) |
| Procedural pain | 7 (19.4) |
| Investigations | |
| Total | 19 (52.8) |
| Blood creatinine increased | 5 (13.9) |
| Vitamin D decreased | 4 (11.1) |
| Metabolic and nutritional disorders | |
| Total | 28 (77.8) |
| Dehydration | 4 (11.1) |
| Diabetes mellitus | 4 (11.1) |
| Gout | 4 (11.1) |
| Hyperglycemia | 12 (33.3) |
| Hyperkalemia | 5 (13.9) |
| Hyperlipidemia | 5 (13.9) |
| Hypokalemia | 7 (19.4) |
| Hypomagnesemia | 20 (55.5) |
| Hypophosphatemia | 11 (30.6) |
| Musculoskeletal and connective tissue disorders | |
| Total | 12 (33.3) |
| Back pain | 5 (13.9) |
| Nervous system disorders | |
| Total | 23 (63.9) |
| Dizziness | 4 (13.1) |
| Headache | 6 (16.7) |
| Tremor | 14 (38.9) |
| Psychiatric disorders | |
| Total | 8 (22.2) |
| Insomnia | 5 (13.9) |
| Renal and urinary disorders | |
| Total | 12 (33.3) |
| Hematuria | 6 (16.7) |
| Reproductive system | |
| Total | 5 (13.9) |
| Scrotal edema/pain | 4 (11.1) |
| Respiratory, thoracic and mediastinal disorders | |
| Total | 15 (41.7) |
| Cough | 5 (13.9) |
| Dyspnea | 4 (11.1) |
| Oropharyngeal pain | 6 (16.7) |
| Skin and subcutaneous tissue | |
| Total | 23 (63.9) |
| Pruritus | 11 (30.6) |
| Urticaria | 6 (16.7) |
| Surgical and medical procedures | |
| Total | 4 (11.1) |
| Incisional drainage | 4 (11.1) |
| Vascular disorders | |
| Total | 18 (50) |
| Hypertension | 11 (30.6) |
| Hypotension | 7 (19.4) |
Adverse event coding was done using the MedDRA Version 13.1 dictionary (http://www.who.int/medical_devices/innovation/Med-DRAintroguide_version14_0_March2011.pdf). Subjects who have the same event more than once are counted only once for the preferred term. Subjects who have more than one adverse event within a system organ class are counted only once in that system organ class. Date produced: October 18, 2013.
Figure 1. Post-TOL101 rashontrunk and abdomen after initial dose.
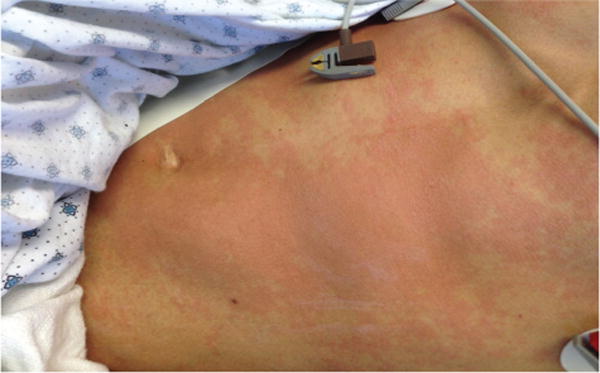
In some cases, TOL101 infusion was associated with a urticarial rash. This is an example of the urticarial rash on the trunk and abdomen of a 22-year-old male treated with 50 mg diphenhydramine and 650 mg acetaminophen.
Table 3.
Urticarial rash incidence; red cells denote TOL101 dose at time of rash
| Treatment Group | Patient | Dose 1 | Dose 2 | Dose 3 | Dose 4 | Dose 5 | Dose 6 | Dose 7 | Dose 8 |
|---|---|---|---|---|---|---|---|---|---|
| 0.28 mg | 06-001 | ||||||||
| 08-001 | |||||||||
| 1.4 mg | 02-001 | ||||||||
| 04-001 | |||||||||
| 7.0 mg | 02-002 | ||||||||
| 04-002 | |||||||||
| 14.0 mg | 05-001 | ||||||||
| 08-002 | |||||||||
| 28.0 mg | 01-001 | ||||||||
| 04-003 | |||||||||
| 07-001 | |||||||||
| 03-001 | |||||||||
| 07-0031 | |||||||||
| 07-004 | |||||||||
| 32.0 mg | 03-0021 | ||||||||
| 08-004 | |||||||||
| 12-0011 | |||||||||
| 07-005 | |||||||||
| 42mg | 06-0021 | ||||||||
| 07-002 | |||||||||
| 04-004 | |||||||||
| 08-003 | |||||||||
| Escalation Group 1 14-42mg | 07-006 | 14mg | 21mg | 28mg | 42mg | 42mg | 42mg | ||
| 07-007 | 14mg | 21mg | 28mg | 42mg | 42mg | 42mg | |||
| 07-008 | 14mg | 21mg | 28mg | 42mg | 42mg | 42mg | |||
| 08-0051 | 14mg | 21mg | 28mg | 42mg | 42mg | 42mg | |||
| 07-009 | 14mg | 21mg | 28mg | 42mg | 42mg | 42mg | |||
| 02-003 | 14mg | 21mg | 28mg | 42mg | 42mg | 42mg | |||
| Escalation Group 2 21-42mg | 07-010 | 21mg | 28mg | 42mg | 42mg | 42mg | 42mg | 42mg | 42mg |
| 07-011 | 21mg | 28mg | 42mg | 42mg | 42mg | 42mg | 42mg | 42mg | |
| 07-012 | 21mg | 28mg | 42mg | 42mg | 42mg | ||||
| 07-013 | 21mg | 28mg | 42mg | 42mg | 42mg | ||||
| 02-004 | 21mg | 28mg | 42mg | 42mg | 42mg | ||||
| 03-003 | 21mg | 28mg | 42mg | 42mg | 42mg | ||||
| 03-004 | 21mg | 28mg | 42mg | 42mg | 42mg |
Patients that had biopsy-confirmed acute rejection
Infections and malignancies
Posttransplant infections observed during the study are presented in Table 4. Only one significant infection was considered potentially associated with TOL101: nosocomial pneumonia detected on chest X-ray. However, no culture was taken from this patient and as such a definitive diagnosis and causative agent cannot be established. Bacterial skin infections were described as incisional wound cellulitis or drainage, with one case of folliculitis reported. Five cases (13.9%) of BKV viremia were reported on protocol viral surveillance at 3 and 6 months. They were treated by immunosuppressive dose reduction according to local practice. There was one case (2.8%) of histologically confirmed BKV associated nephropathy (Patient 8–005). These six cases (16.7%) were spread across three dosing cohorts: Group 4, 14 mg = 1; Group 6, 32 mg = 2 and Group 8, dose-escalating chort 14–42 mg = 3, and as such a dose–response is not directly apparent. One case of CMV viremia with colitis occurred at Day 169. No EBV or pneumocystis pneumonia was observed. No malignancies have been reported to date in subjects who received TOL101.
Table 4.
Infections and malignancies (N = 36)
| Infections | Patients, n (%) |
|---|---|
| Bacterial | |
| Skin | 5 (13.9) |
| Pneumonia | 1 (2.8) nosocomial |
| Pharyngitis, rhinitis and sinusitis | 5 (13.9) |
| Urinary tract | 3 (8.3) |
| Urosepsis | 1 (2.8) Escherichia coli |
| Viral (viremia) | |
| CMV | 2 (5.6) |
| BK | 5 (13.9) |
| BK associated nephropathy | 1 (2.8) |
| EBV | 0 (0) |
| Fungal | |
| Candida | 0 (0) |
| Other | 0 (0) |
| Opportunistic | |
| Pneumocystis | 0 (0) |
| Cancer | |
| PTLD | 0 (0) |
| Solid organ | 0 (0) |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; PTLD, posttrans-plant lymphoproliferative disorder.
Cytokine release and anti-drug antibody detection
As noted in the AE tables, symptoms associated with cytokine release syndrome were not observed. The lack of symptoms was supported by the low levels of IFN-γ, TNF, IL-2, IL-6 and IL-10 at baseline and 0, 2, 4, 8 and 24 h after infusion, respectively (Figure 2A–E). Furthermore, another potential inflammatory marker indicative of infusion reactions includes the production of NO, which was not significantly detected in TOL101-treated patients during or after infusions on Days 0 and 4. The mean percent change from baseline NO levels in micromolar during infusion, end of infusion and 2 h. Postinfusion were −24%, −2%, −19%, +12%, −31% and +4% for cohort Groups 4–9, respectively. Low titer HAMA (1/100) was detected in only 1/36 (2.8%) patient on Day 28. No high-titer HAMA (1/1000) was ever detected.
Figure 2. The production of cytokines after the first dose of TOL101.
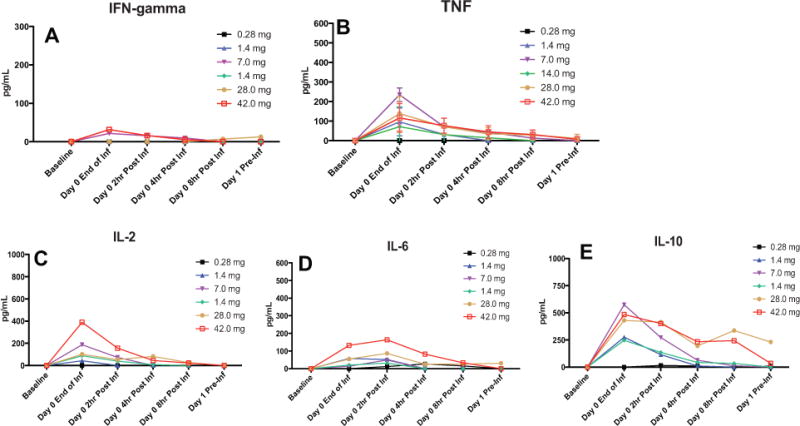
(A–E) The production of IFN-γ (A), TNF (B), IL-2 (C), IL-6(D)and IL-10 (E) was measured 0, 2, 4, 8 and 24 h after the first infusion of TOL101. No difference in cytokine responses was observed across dose levels. The mean ± standard deviation are presented. IFN-γ, interferon-γ; TNF, tumor necrosis factor.
Efficacy as measured by CD3 count reduction
Peripheral blood CD3 T cell counts were measured daily in the morning within 1–2h of the next TOL101 infusion. During TOL101 treatment, dosing of TOL101 was required for a minimum of 5 days (six doses), with continued dosing until therapeutic tacrolimus levels were reached (8–15 ng/mL). In seven patients, further doses were needed. In all patients, a primary reduction in leukocyte counts, including CD3 expressing cells was observed immediately after transplant, a potential result of IV steroid infusion (24). Within 48–72h circulating CD3 counts increased above the 25/mm3 target in patients receiving 0.28, 2.4, 7 and 14mg TOL101 (Figure 3A). In the 28 mg cohort, CD3 counts remained mostly under 25/mm3, with the exception of one patient who experienced a spike in CD3 numbers on Day 3 (Figure 3B). While 28 mg appeared to be a promising dose regimen, this one outlier triggered escalation of TOL101 to 32 and 42 mg. At both dosing regimens robust CD3 modulation was achieved; however, a rash was noted in patients in the 32 and 42 mg cohorts, respectively. To address possible preformed T cell soluble mediator release as a mechanism, two dose-escalation strategies were tested to exhaust these stores at sub-symptomatic levels. One strategy began at 14 mg on Day 0 with the other at 21 mg on Day 0. In each case, the dose was rapidly escalated to 42 mg by the third or fourth dose. The utilization of a dose-escalating regimen not only reduced the propensity for rash development but also resulted in robust CD3 modulation, meeting the PD target.
Figure 3.
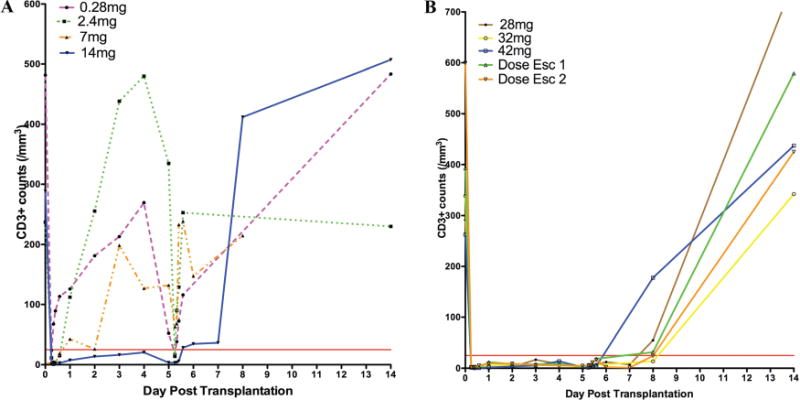
(A, B) Pharmacodynamic (CD3 absolute/mm3) responses to increasing dosesofTOL101 over 5–9 days. The mean of each cohort is presented.
The recovery of CD3 expression after TOL101 dosing was observed to occur in all patients by Day 14 (Figure 3B). This recovery combined with stable white blood cell counts suggests the possibility of a nondepletional mechanism of action. The mean plasma elimination half-life of TOL101 in patients receiving greater than 28 mg or in the dose-escalating cohorts was 23.8 ± 9 h, supporting once daily infusions.
Efficacy composite triple end point
There were no patient deaths or graft losses reported in the study, and five (13.9%) subjects experienced BCAR episodes (Table 5). While no rejections occurred during TOL101 treatment, their occurrence was relatively soon after the drug was completed on Days 10–20, and three had a vascular component. The last tacrolimus trough blood level (ng/mL) prior to each rejection was Day 10 (13.1), Day 11 (7.8), Day 14 (6.3), Day 16 (7.6) and Day 20 (8.6). All rejection episodes were treated with thymoglobulin (4) or steroids (1), and resolved clinically without graft loss. No donor-specific anti-HLA antibodies have been detected in the 22 patients tested at both 3 and 6 months posttrans-plant. Fourteen patients were not tested.
Table 5.
Efficacy observations: N = 36 (end of study 6 months)
| End point | N (%) |
|---|---|
| Patient survival | 36 (100) |
| Graft survival | 36 (100) |
| Treated BCAR | 5 (13.9) |
| Banff scored | |
| 1A (Day 10) | 1 |
| 1B (Day 16) | 1 |
| 2A (Days 11, 14 and 20) | 3 |
| 2B or 3 | 0 |
| Graft function | |
| Delayed graft function (all), n ¼36 | 2 (5.6) |
| Delayed graft function (deceased donors), n = 8 | 2 (25) |
| Estimated GFR (cc/min) (Day 180), n¼36 | 55.6±10 |
| Measured GFR (cc/min/1.73m2) (Day 180), n = 23 | 65.7 ±26 |
| Urine protein/creatinine | |
| (Day 90) | 0.17 ±0.12 |
| (Day 180) n¼27 | 0.13±0.11 |
| Donor-specific antibody | |
| n = 22 tested; 14 not tested | |
| Day 90 | 0 |
| Day 180 | 0 |
BCAR, biopsy-confirmed acute rejection.
Kidney function
Two patients who received ECD kidneys in cohort 9 (07–010 and 07–011; Table 3) required first week dialysis. In general, kidney function was observed to improve throughout the study with increases in eGFR observed in all patients (Day 180 mean eGFR being 54 mg/dL) (Table 5). In addition, of the 23 subjects who completed a measured (iothalamate) GFR the mean was 65.7 mL/min/1.73 m2. A voided urine protein to creatinine ratio was performed on Day 90 (n = 27) and Day 180/EOS (n = 27). Excluding three patients due to laboratory handling issues, ratios observed were 0.17 ± 0.12 and 0.13 ± 0.11, respectively (Table 5).
Discussion
Targeting the αβ TCR with TOL 101 is hypothesized to provide rapid and robust T cell modulation, low acute rejection rates and reduced AE compared with currently utilized induction agents. The results from this first-in-human Phase 2 clinical trial investigating the safety and efficacy of TOL101 in 36 primary renal transplant patients are reported. In particular, advancing from subtherapeutic to therapeutic dosing regimens did not result in diminished clinical outcomes or untoward events. These data show that TOL101 is a highly TCR-specific mAb capable of providing robust T cell modulation, without inducing significant cytokine release and/or other immunologic toxicity.
TOL101 was generally well tolerated across all groups, with urticarial rash the only significant AE resulting in study drug discontinuation in three subjects. Skin eruptions are commonly reported side effects in patients receiving biologics, and have been observed in some patients receiving rabbit ATG, alemtuzumab and anti-CD3 agents (25). In the case of TOL101, rash was not associated with any hemodynamic effects, skin breakdown or long-lasting sequelae. Cytokine release and anaphylaxis are potential causes of rash; however, no patient in the current study appeared to have any anaphylactoid like reaction. The levels (pg/mL) of the examined pro-inflammatory cytokines IFN-γ, TNF, IL-2, IL-6 and IL-10 were relatively low in all TOL101-treated patients (Figure 2A–E). In comparison, infusion of rabbit ATG is accompanied by release at levels of 1000–3000 pg/mL of these cytokines (14). While the specific etiology of the rash is under further examination, one possible explanation for the rash was T cell release of preformed nonclassical soluble mediators with resultant cutaneous vasodilation. As such a dose-escalation strategy combined with a slower infusion rate was used to deplete these potential granule stores. This was viewed to be successful with patients receiving an escalating regimen of 21, 28, 42, 42 and 42 mg experiencing minimal rash symptoms, permitting the full infusion with the 42mg dose.
Historically, other anti-αβ TCR antibodies have been tested in humans, namely T10B9 and BMA-031. Not only both these agents showed promising efficacy, but their safety profiles were also favorable, with less cytokine release syndrome and serious infections reported compared to muromonab-CD3 (21–23). However, the incidence and intensity of HAMA formation after treatment were similar to those recorded for muromonab-CD3. Unlike these predecessor antibodies, whereby immunogenicity was a major drawback, TOL101 utilization has been associated with very little anti-drug antibody formation, a result of different manufacturing processes and contemporary immune suppression (15,25,26). In this Phase 2 trial, only 1/36 (2.8%) of treated recipients demonstrated HAMA formation, a similar incidence to the chimerized IL-2 receptor blocker basiliximab, 1.5% (27). This potentially makes αβ TCR blockade an attractive target for the pharmacologic control of autoimmunity and transplant rejection (28).
This initial short-term (6-month) experience using TOL101 induction with tacrolimus, MMF and low-dose steroids in clinical kidney transplantation demonstrated excellent patient and graft survival with acceptable AE rates similar to conventional therapies. Together these initial data show TOL101 to be relatively safe and highly effective at modulating CD3+ T cells. This low to moderate risk population experienced a BCAR rate of 13.9%, with the majority of episodes occurring within the first 3 weeks of transplant. In addition, TOL101 induction permitted stable renal function and no reports of DSA development for up to 6 months. The plasma elimination half-life of 23.8 h permits once daily administration, and the apparent recovery of circulating CD3 T cells by 14 days suggests a narrower window of intense immunosuppression than other biologics. The rate of recovery of TOL101 treated T cells and their function should be an active area of future investigation. Finally, and when taken together, these data support the initiation of larger Phase 3 studies testing TOL101 against thymoglobulin and basiliximab, using the final dose-escalating regimen (cohort 9).
Supplementary Material
Table S1: All reported serious adverse events.
Acknowledgments
This study was funded by Tolera Therapeutics, Inc. The authors would like to thank Dr. Maria Siemionow for her important role in the early development of TOL101 as an immunosuppressive agent.
Abbreviations
- AE
adverse events
- ATG
anti-thymocyte globulin
- BCAR
biopsy-confirmed acute rejection
- BKV
BK virus
- CMV
cytomegalovirus
- DSA
donor-specific antibody
- DSMB
data safety monitoring board
- EBV
Epstein-Barr virus
- ECD
expanded criteria deceased donor
- eGFR
estimated GFR
- ELISA
enzyme-linked immunosorbent assay
- EOS
end of study
- HAMA
human anti-mouse antibody
- IFN-γ
interferon-γ
- MABEL
minimum anticipated biologic effect level
- MMF
mycophenolate mofetil
- NO
nitric oxide
- PD
pharmacodynamic
- SAE
serious adverse events
- TNF
tumor necrosis factor
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. JPP, JJH, DRG, MTG, TJF, LO and FF are employees of and have stock holdings with Tolera. ACW is an employee of Tolera. SMF, LBM, SM, THW, AA, RS, SDM and FS are scientific advisors for Tolera.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 2.Dierselhuis M, Goulmy E. The relevance of minor histocompatibility antigens in solid organ transplantation. Curr Opin Organ Transplant. 2009;14:419–425. doi: 10.1097/MOT.0b013e32832d399c. [DOI] [PubMed] [Google Scholar]
- 3.Archbold JK, Ely LK, Kjer-Nielsen L, et al. T cell allorecognition and MHC restriction—A case of Jekyll and Hyde? Mol Immunol. 2008;45:583–598. doi: 10.1016/j.molimm.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 5.Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 6.Flechner SM. Sirolimus in kidney transplantation indications and practical guidelines: De novo sirolimus based therapy without calcineurin inhibitors. Transplantation. 2009;87:S1–S6. doi: 10.1097/TP.0b013e3181a059a1. [DOI] [PubMed] [Google Scholar]
- 7.Webster AC, Playforde G, Higgins G, Chapman JR, Craig JC. Interleukin 2 receptor antagonists for renal transplant recipients: A meta-analysis of randomized trials. Transplantation. 2004;77:166–176. doi: 10.1097/01.TP.0000109643.32659.C4. [DOI] [PubMed] [Google Scholar]
- 8.Patlola V, Zhong X, Reed GW, Mandelbrot DA. Efficacy of anti-IL-2 receptor antibodies compared to no induction and to antilymphocyte antibodies in renal transplantation. Am J Transplant. 2007;7:1832–1841. doi: 10.1111/j.1600-6143.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 9.Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: An analysis of United Network for Organ Sharing registry data. Transplantation. 2010;90:1511–1515. doi: 10.1097/TP.0b013e3181fecfcb. [DOI] [PubMed] [Google Scholar]
- 10.Nashan B. Antibody induction therapy in renal transplant patients receiving calcineurin-inhibitor immunosuppressive regimens. Bio-drugs. 2005;19:39–46. doi: 10.2165/00063030-200519010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Wagner SJ, Brennan DC. Induction therapy in renal transplant recipients: How convincing is the current evidence? Drugs. 2012;72:671–683. doi: 10.2165/11631300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Brennan DC, Daller JA, Lake KD, et al. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 13.Hanaway MJ, Woodle ES, Mulgaonkar S, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364:1909–1919. doi: 10.1056/NEJMoa1009546. [DOI] [PubMed] [Google Scholar]
- 14.Guttmann RD, Caudrelier P, Alberici G, Touraine JL. Pharmacokinetics, foreign protein immune response, cytokine release, and lymphocyte subsets in patients receiving thymoglobuline and immunosuppression. Transplant Proc. 1997;29:24S–26S. [PubMed] [Google Scholar]
- 15.Broeders N, Wissing KM, Crusiaux A, Kinnaert P, Vereerstraeten P, Abramowicz D. Mycophenolate mofetil, together with cyclo-sporin A, prevents anti-OKT3 antibody response in kidney transplant recipients. J Am Soc Nephrol. 1998;9:1521–1525. doi: 10.1681/ASN.V981521. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman DB, Leventhal JR, Axelrod D, et al. Alemtuzumab induction and prednisone-free maintenance immunotherapy in kidney transplantation: Comparison with basiliximab induction— Long-term results. Am J Transplant. 2005;5:2539–2548. doi: 10.1111/j.1600-6143.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto Y, Tsuchida M, Hanawa H, Abo T. Successful prevention and treatment of autoimmune encephalomyelitis by short-term administration of anti-T-cell receptor alpha beta antibody. Immunology. 1994;81:1–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Sempe P, Bedossa P, Richard MF, Villa MC, Bach JF, Boitard C. Anti-alpha/beta T cell receptor monoclonal antibody provides an efficient therapy for autoimmune diabetes in nonobese diabetic (NOD) mice. Eur J Immunol. 1991;21:1163–1169. doi: 10.1002/eji.1830210511. [DOI] [PubMed] [Google Scholar]
- 19.Khariwala SS, Knott PD, Dan O, Klimczak A, Siemionow M, Strome M. Pulsed immunosuppression with everolimus and anti-alpha-beta T-cell receptor: Laryngeal allograft preservation at six months. Ann Otol Rhinol Laryngol. 2006;115:74–80. doi: 10.1177/000348940611500111. [DOI] [PubMed] [Google Scholar]
- 20.Siemionow MZ, Izycki DM, Zielinski M. Donor-specific tolerance in fully major histocompatibility major histocompatibility complex-mismatched limb allograft transplants under an anti-alphabeta T-cell receptor monoclonal antibody and cyclosporine A protocol. Transplantation. 2003;76:1662–1668. doi: 10.1097/01.TP.0000105343.49626.6F. [DOI] [PubMed] [Google Scholar]
- 21.Waid TH, Lucas BA, Thompson JS, et al. Treatment of renal allograft rejection with T10B9.1A31 or OKT3: Final analysis of a phase II clinical trial. Transplantation. 1997;64:274–281. doi: 10.1097/00007890-199707270-00017. [DOI] [PubMed] [Google Scholar]
- 22.Waid TH, Lucas BA, Thompson JS, et al. Treatment of acute cellular rejection with T10B9.1A-31 or OKT3 in renal allograft recipients. Transplantation. 1992;53:80–86. doi: 10.1097/00007890-199201000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Beelen DW, Graeven U, Schulz G, et al. Treatment of acute graft-versus-host disease after HLA-partially matched marrow transplantation with a monoclonal antibody (BMA031) against the T cell receptor. First results of a phase-I/II trial. Onkologie. 1988;11:56–58. doi: 10.1159/000216484. [DOI] [PubMed] [Google Scholar]
- 24.Haynes BF, Fauci AS. The differential effect of in vivo hydrocortisone on the kinetics of subpopulations of human peripheral blood thymus-derived lymphocytes. J Clin Invest. 1978;61:703–707. doi: 10.1172/JCI108982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Getts DR, Getts MT, McCarthy DP, Chastain EM, Miller SD. Have we overestimated the benefit of human(ized) antibodies? MAbs. 2010;2:682–694. doi: 10.4161/mabs.2.6.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flechner SM, Goldfarb DA, Fairchild R, et al. A randomized prospective trial of low dose OKT3 to prevent rejection and minimize side effects in recipients of kidney transplants. Transplantation. 2000;69:2374–2381. doi: 10.1097/00007890-200006150-00027. [DOI] [PubMed] [Google Scholar]
- 27.Nashan B, Moore R, Amlot P, et al. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. Lancet. 1997;350:1193–1198. doi: 10.1016/s0140-6736(97)09278-7. [DOI] [PubMed] [Google Scholar]
- 28.Getts DR, Shankar S, Chastain EM, et al. Current landscape for T-cell targeting in autoimmunity and transplantation. Immunotherapy. 2011;3:853–870. doi: 10.2217/imt.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: All reported serious adverse events.


