Abstract
An isolated “native” photosystem I (PSI complex) contains three spectral populations of chlorophyll a antennae (Mullet, Burke, Arntzen 1980 Plant Physiol 65: 814-822). It was hypothesized that nearly one-half of these antennae (≃45 Chl/P700) are associated with polypeptides of 21,500 to 24,500 daltons. The present study utilizes two developmental systems to verify this association.
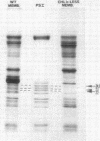
Chloroplasts were isolated from a Chl b-less barley mutant and from partially-developed cucumber cotyledons (greened under intermittent illumination [ImL] chloroplasts) and were compared to control chloroplasts isolated from wild-type barley and mature cucumber. Both the mutant and ImL chloroplasts exhibited a long wavelength fluorescence maximum at 724 nanometers at 77 K as compared to 735 to 738 nanometers emission maximum in the respective controls. Both the mutant and ImL chloroplasts were deficient in polypeptides of 21,500 to 24,500 daltons which were present in control membranes and in PSI fractions isolated from control membranes. In light-induced maturation of the ImL cucumbers, the synthesis of polypeptides in the 21,500 to 24,500 molecular weight range paralleled the appearance of PSI Chl species fluorescing at long wavelength (≃735 nm).
The PSI spectral properties of the control membranes were retained in isolated PSI particles containing 100 to 120 Chl/P700 (PSI-110). Detergent extraction of PSI-110 removed polypeptides of 21,500 to 24,500 daltons plus ≃ 45 Chl/P700. The antennae-depleted PSI particle mimics PSI properties exhibited by incompletely differentiated mutant or ImL chloroplasts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armond P. A., Arntzen C. J., Briantais J. M., Vernotte C. Differentiation of chloroplast lamellae. Light harvesting efficiency and grana development. Arch Biochem Biophys. 1976 Jul;175(1):54–63. doi: 10.1016/0003-9861(76)90484-7. [DOI] [PubMed] [Google Scholar]
- Armond P. A., Staehelin L. A., Arntzen C. J. Spatial relationship of photosystem I, photosystem II, and the light-harvesting complex in chloroplast membranes. J Cell Biol. 1977 May;73(2):400–418. doi: 10.1083/jcb.73.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen C. J., Ditto C. L. Effects of cations upon chloroplast membrane subunit. Interactions and excitation energy distribution. Biochim Biophys Acta. 1976 Nov 9;449(2):259–274. doi: 10.1016/0005-2728(76)90138-9. [DOI] [PubMed] [Google Scholar]
- Bar-Nun S., Schantz R., Ohad I. Appearance and composition of chlorophyll-protein complexes I and II during chloroplast membrane biogenesis in Chlamydomonas reinhardi y-1. Biochim Biophys Acta. 1977 Mar 11;459(3):451–467. doi: 10.1016/0005-2728(77)90045-7. [DOI] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Boardman N. K., Highkin H. R. Studies on a barley mutant lacking chlorophyll b. I. Photochemical activity of isolated chloroplasts. Biochim Biophys Acta. 1966 Oct 10;126(2):189–199. doi: 10.1016/0926-6585(66)90054-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Steinback K. E., Arntzen C. J. Analysis of the Light-harvesting Pigment-Protein Complex of Wild Type and a Chlorophyll-b-less Mutant of Barley. Plant Physiol. 1979 Feb;63(2):237–243. doi: 10.1104/pp.63.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. J., Armond P. A., Gross E. L., Arntzen C. J. Differentiation of chloroplast lamellae. Onset of cation regulation of excitation energy distribution. Arch Biochem Biophys. 1976 Jul;175(1):64–70. doi: 10.1016/0003-9861(76)90485-9. [DOI] [PubMed] [Google Scholar]
- Gasanov R. A., French C. S. Chlorophyll composition and photochemical activity of photosystems detached from chloroplast grana and stroma lamellae. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2082–2085. doi: 10.1073/pnas.70.7.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller R. G., Pilger T. B., Genge S. Effect of linocmycin on the chlorophyll protein complex I content and photosystem I activity of greening leaves. Biochim Biophys Acta. 1977 Jun 9;460(3):431–444. doi: 10.1016/0005-2728(77)90082-2. [DOI] [PubMed] [Google Scholar]
- Ikegami I. Fluorescence changes related in the primary photochemical reaction in the P-700-enriched particles isolated from spinach chloroplasts. Biochim Biophys Acta. 1976 Nov 9;449(2):245–258. doi: 10.1016/0005-2728(76)90137-7. [DOI] [PubMed] [Google Scholar]
- Kung S. D., Thornber J. P., Wildman S. G. Nuclear DNA codes for the photosystem II chlorophyll-protein of chloroplast membranes. FEBS Lett. 1972 Aug 1;24(2):185–188. doi: 10.1016/0014-5793(72)80763-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Butler W. L. Low temperature spectral properties of subchloroplast fractions purified from spinach. Plant Physiol. 1978 Mar;61(3):373–379. doi: 10.1104/pp.61.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K., Mathis P., Acker S., van Best J. A. Electron acceptors associated with P-700 in Triton solubilized photosystem I particles from spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):120–134. doi: 10.1016/0005-2728(78)90166-4. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]