Abstract
Congenital heart disease (CHD), the most common type of birth defect, is still the leading non-infectious cause of infant morbidity and mortality in humans. Aggregating evidence demonstrates that genetic defects are involved in the pathogenesis of CHD. However, CHD is genetically heterogeneous and the genetic components underpinning CHD in an overwhelming majority of patients remain unclear. In the present study, the coding exons and flanking introns of the PITX2 gene, which encodes a paired-like homeodomain transcription factor 2essential for cardiovascular morphogenesis as well as maxillary facial development, was sequenced in 196 unrelated patients with CHD and subsequently in the mutation carrier’s family members available. As a result, a novel heterozygous PITX2 mutation, p.Q102X for PITX2a, or p.Q148X for PITX2b, or p.Q155X for PITX2c, was identified in a family with endocardial cushion defect (ECD) and Axenfeld-Rieger syndrome (ARS). Genetic analysis of the pedigree showed that the nonsense mutation co-segregated with ECD and ARS transmitted in an autosomal dominant pattern with complete penetrance. The mutation was absent in 800 control chromosomes from an ethnically matched population. Functional analysis by using a dual-luciferase reporter assay system revealed that the mutant PITX2 had no transcriptional activity and that the mutation eliminated synergistic transcriptional activation between PITX2 and NKX2.5, another transcription factor pivotal for cardiogenesis. To our knowledge, this is the first report on the association of PITX2 loss-of-function mutation with increased susceptibility to ECD and ARS. The findings provide novel insight into the molecular mechanisms underpinning ECD and ARS, suggesting the potential implications for the antenatal prophylaxis and personalized treatment of CHD and ARS.
Introduction
Congenital heart disease (CHD) is the most prevalent type of birth defect in humans, with an estimated prevalence of 1% among living neonates, and is the most common non-infectious cause of infant morbidity and mortality, accounting for roughly 30% of neonatal demises caused by miscellaneous developmental malformations [1]. Traditionally, various CHDs are categorized as at least 21 distinct entities with specific anatomic lesions, including ventricular septal defect, atrial septal defect, tetraology of Fallot, endocardial cushion defect (ECD), double outlet right ventricular, patent ductus arteriosus, and transposition of the great vessels [1]. Distinct forms of CHDs can occur separately or in combination, leading to reduced exercise performance, degraded quality of life, delayed brain development or brain injury, thromboembolic stroke, pulmonary hypertension, impaired pulmonary function, metabolic disorders, muscle dysfunction, abnormal autonomic nervous activity, infective endocarditis, cardiac enlargement or congestive heart failure, arrhythmias, and sudden cardiac death [2–13]. Obviously, CHD has imposed an enormous economic burden on patients and health care systems, and the socioeconomic burden is anticipated to increase in the future with increasing CHD adults [14,15]. Despite the pronounced clinical importance, the molecular mechanisms underpinning CHD remain poorly understood.
In vertebrates, the heart is the first organ that develops to function. Cardiovascular morphogenesis is a complex, dynamic biological process that requires the orchestration of cardiac cell commitment, differentiation, proliferation and migration, and both environmental and genetic risk factors may interrupt this accurate temporal and spatial cooperation, yielding a wide range of CHD [16–38]. There is increasing evidence that highlights the pivotal role of cardiac transcription factors in embryonic cardiogenesis, and a long list of mutations in the cardiac transcription factor genes, including NK and GATA families, have been implicated in the pathogenesis of CHD [39–65]. However, CHD is of striking genetic heterogeneity and the genetic components predisposing to CHD in an overwhelming majority of patients remain to be identified.
Recently, there is increasing evidence demonstrating that the transcription factor PITX2, a member of the bicoid-like homeodomain family of transcription factors, plays a crucial role in cardiovascular morphogenesis and maxillary facial development. The PITX2 gene was originally identified as a causative gene for the human Axenfeld-Rieger's syndrome (ARS), which is characterized by eye, teeth, craniofacial and umbilical abnormalities as well as heart defects [66–68]. To date, four different isoforms of PITX2 transcripts, which are generated by differential mRNA splicing and alternative promoter usage, have been identified, of which PITX2a, PITX2b and PITX2c differ only in their amino-termini and exist in human, mouse, chick, zebrafish and xenopus, while the fourth isoform, PITX2d, which lacks most homeodomain along with the entire amino-terminal domain, is detected only in humans. Notably, PITX2c is the predominant transcript in the embryonic and adult heartsof the mouse and human, mainly responsible for cardiogenesis [69–78]. In Xenopus embryos, partial depletion of PITX2c mRNA using chemically modified antisense oligonucleotides resulted in cardiac dysmorphology, including abnormalities of outflow tract, atrial septation and relative atrial-ventricular chamber positioning as well as restriction of ventricular development [79]. In mice, targeted disruption of PITX2c resulted in embryonic lethality with different kinds of congenital cardiovascular malformations, including ECD, atrial isomerism, double-outlet right ventricle, transposition of the great artery and abnormal aortic arch [80,81]. In humans, PITX2cmutations have been causatively associated with isolated congenital heart diseases [82–84]. These findings justified screening PITX2 as a preferred candidate gene for CHD in other cohorts of patients.
Materials and Methods
Study participants
In this study, 196 unrelated CHD patients and 400 unrelated individuals with no cardiac structural aberrations were enrolled from the Chinese Han population. The available relatives of an index patient with an identified PITX2 mutation were also included. All participants underwent detailed clinical evaluation, which included individual and familial histories, comprehensive physical examination, and echocardiography with color flow Doppler. The patients also underwent chest X-ray, electrocardiogram or cardiac catheterization examination when there was a strong clinical indication. Medical records of the deceased or unavailable relatives of a mutation carrier were also reviewed. The patients with known chromosomal abnormalities were excluded from the study. Peripheral venous blood samples were taken from all participants. This study conformed to the ethical guidelines of the Declaration of Helsinki. The study protocol was reviewed and approved by the ethics committee of Tongji Hospital, Tongji University (the ethical approval number for cases and controls: LL(H)-09-07; the date for the approval: July 27, 2009). Written informed consent was signed by participants or their guardians prior to study.
Genetic analysis of human PITX2
Genomic DNA was isolated from peripheral blood leukocytes using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). The coding regions and splice junction sites of the PITX2 gene was sequenced initially in 196 unrelated patients with CHD, and genotyping PITX2 was performed subsequently in the available relatives of a mutation carrier and 400 unrelated control individuals. The referential genomic DNA sequence of PITX2 was derived from GenBank (accession no. NC_000004), which was at the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/). The primer pairs used to amplify the coding exons and intron–exon boundaries of PITX2 by polymerase chain reaction (PCR) were shown in Table 1. The PCR was performed and the PCR product was sequenced as previously described [82]. A sequence variation was verified by re-sequencing an independent PCR-amplified product from the same subject. Additionally, for an identified sequence variant, the Exome Variant Server (EVS; http://evs.gs.washington.edu/EVS) and NCBI’s single nucleotide polymorphism (SNP; http://www.ncbi.nlm.nih.gov/SNP) databases were queried to confirm its novelty.
Table 1. The primers to amplify the coding exons and flanking introns of PITX2.
| Exon | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Amplicon (bp) |
|---|---|---|---|
| 2 | GAGGCTAGGCTGGAGATGCT | CCACTGGCGATTTGGTTCTG | 385 |
| 3 | TTGCTCTTTGTCCCTCTTTC | CCAGAGGCGGAGTGTCTAAG | 399 |
| 4 | CAGCTTGGCTTGAGAACTCG | TGACTTCCTTGGGGCGAGAG | 442 |
| 5 | CAGCTCTTCCACGGCTTCTG | GCTGCCTTCCACATTCTCTC | 387 |
| 6 | AATCTGCACTGTGGCATCTG | AGTCTTTCAAGGGCGGAGTT | 677 |
Alignment of multiple PITX2 protein sequences across species
Multiple amino acid sequences of the PITX2 proteins from various species were aligned using the online MUSCLE program, version 3.6 (http://www.ncbi.nlm.nih.gov/).
Plasmids and site-directed mutagenesis
The expression plasmid PITX2c-pcDNA4 was a kind gift from Georges Christé at Physiopathologie des Troubles du RythmeCardiaque, Faculté de Pharmacie de Lyon, Université Lyon 1, France. The recombinant expression plasmid NKX2.5-pEFSA and the atrial natriuretic factor (ANF)-luciferase reporter plasmid (ANF-luc), which contains the 2600-bp 5’-flanking region of the ANF gene and expresses Firefly luciferase, were kindly provided by Dr. Ichiro Shiojima, from the Department of Cardiovascular Science and Medicine, Chiba University Graduate School of Medicine, Chuo-ku, Chiba, Japan. The procollagen lysyl hydroxylase (PLOD1) promoter plasmid PLOD1-luc, which contains the nucleotides from -60 to -3180 of the PLOD1 gene, was constructed as described previously [85]. The PITX2a and PITX2b isoforms were PCR-amplified from cDNA clones as described previously [86] and inserted into the pcDNA4 plasmid (Invitrogen, Carlsbad, CA, USA), respectively. The identified mutation Q102X, or Q148X, or Q155X was introduced into the wild-type PITX2a, or PITX2b, or PITX2c, respectively, by using a QuickChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) with a complementary pair of primers. Each of themutants was sequenced to confirm the desired mutation and to exclude any other sequence variations.
Luciferase reporter gene assays
Chinese hamster ovary (CHO) cells were seeded in 12-well plates and cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, 100 mg/ml penicillin, and 100 mg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. Cell transfections were performed 24 h after plating, with Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer’s protocol. The ANF-luc construct and an internal control reporter plasmid pGL4.75 (hRluc/CMV, Promega), which expresses Renilla luciferase, were used in transient transfection assays. CHO cells were transfected with 2 μg of wild-type PITX2–pcDNA4 or mutant PITX2–pcDNA4 or empty vector pcDNA4, 2.0 μg of ANF-luc reporter construct, and 0.04 μg of pGL4.75 control reporter vector. For co-transfection experiments, 1 μg of wild-type PITX2–pcDNA4, 1 μg of mutant PITX2–pcDNA4, 2.0 μg of ANF-luc, and 0.04 μg of pGL4.75 were used. Transfected cells were harvested 24 h after transfection, then lysed and assayed for reporter activities. Firefly luciferase and Renilla luciferase activities were measured with the Dual-Glo luciferase assay system (Promega). The activity of the ANF promoter was presented as fold activation of Firefly luciferase relative to Renilla luciferase. Three independent experiments were conducted in triplicate for wild-type and mutant PITX2a, or PITX2b, or PITX2c, and results are representative of three separate experiments.
For the analysis of the synergistic transcriptional activation between PITX2 and NKX2.5 [87], another transcription factor crucial for normal cardiovascular development [40–48], CHO cells were grown and transfected with 2μg of wild-type or mutant PITX2–pcDNA4, alone or together with 2μg of wild-type NKX2.5-pEFSA, 5μg of PLOD1-luc, and 0.04 μg of pGL4.75 using Lipofectamine 2000 Transfection Reagent (Invitrogen).
Statistical analysis
The significance of differences in luciferase activity was analyzed using the unpaired Student’s t test. A two-tailed P value less than 0.05 was considered to be statistically significant.
Results
Baseline characteristics of the study subjects
A cohort of 196 unrelated patients with CHD was clinically investigated in contrast to a total of 400 ethnically-matched unrelated controls. All the participants had no established environmental risk factors for CHD, such as maternal illness and drug use in the first trimester of pregnancy, parental smoking and long-term exposure to toxicants as well as ionizing radiation. The control individuals had no evidence of organic cardiac diseases, and their echocardiographic results were normal. The baseline clinical characteristics of the 196 CHD patients are summarized in Table 2.
Table 2. Baseline clinical characteristics of the 196 unrelated patients with congenital heart disease.
| Variable | Statistic |
|---|---|
| Male gender (%) | 102 (52.0) |
| Age (years) | 5.2 ± 2.4 |
| Positive family history (%) | 36 (18.4) |
| Prevalence of different types of CHD | |
| Isolated CHD (%) | 105 (53.6) |
| VSD (%) | 32 (16.3) |
| ASD (%) | 27 (13.8) |
| PDA (%) | 20 (10.2) |
| ECD (%) | 6 (3.1) |
| AS (%) | 5 (2.6) |
| PA (%) | 5 (2.6) |
| CoA (%) | 4 (2.0) |
| PS (%) | 3 (1.5) |
| TA | 2 (1.0) |
| HLHS (%) | 1 (0.5) |
| Complex CHD (%) | 72 (36.7) |
| TOF (%) | 28 (14.3) |
| DORV + VSD (%) | 17 (8.7) |
| ECD + TGA (%) | 14 (7.1) |
| TA + VSD (%) | 9 (4.6) |
| TGA +VSD (%) | 4 (2.0) |
| Others (%) | 19 (9.7) |
| Incidence of arrhythmia | |
| Atrial fibrillation (%) | 16 (8.2) |
| Atrioventricular block (%) | 8 (4.1) |
| Treatment | |
| Surgical repair (%) | 118 (60.2) |
| Catheter-based closure (%) | 57 (29.1) |
| Follow-up (%) | 21 (10.7) |
CHD, congenital heart disease; VSD, ventricular septal defect; ASD, atrial septal defect; PDA, patent ductus arteriosus; ECD, endocardial cushion defect; AS, aortic stenosis; PA, pulmonary atresia; CoA, coarctation of the aorta; PS, pulmonary stenosis; TA, truncusarteriosus; HLHS, hypoplastic left heart syndrome; TOF, tetralogy of Fallot; DORV, double outlet of right ventricle; TGA, transposition of great arteries.
Identification of a novel PITX2 mutation
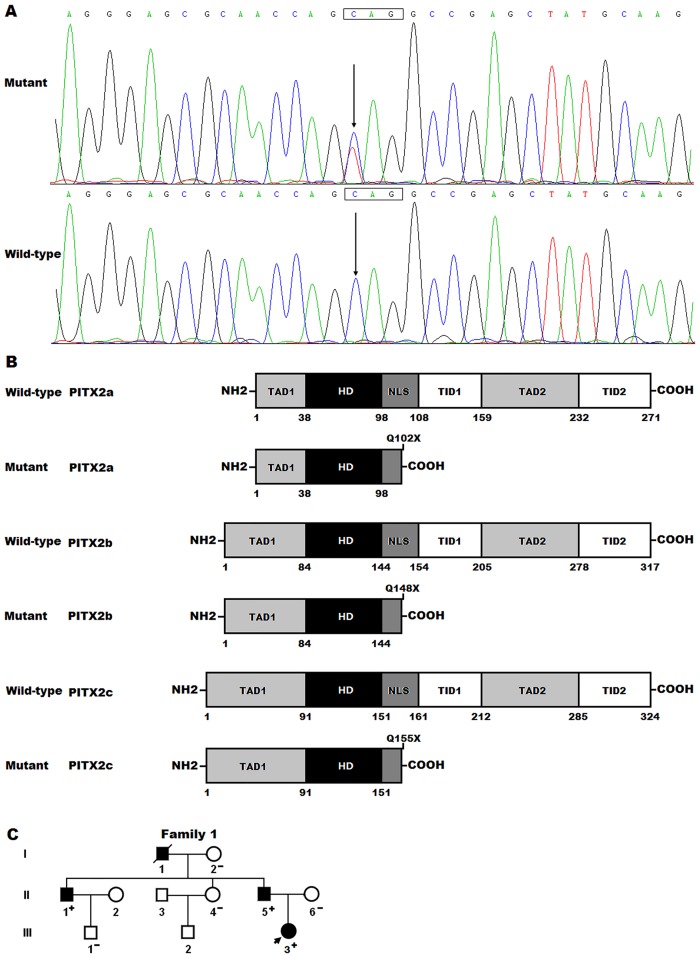
By sequencing of PITX2 in the 196 patients, a heterozygous sequence variation was identified in one patient, with a mutational prevalence of about 0.51%. Specifically, a substitution of thymine for cytosine at the first nucleotide of codon 102 of PITX2a (c.304C>T), or codon 148 of PITX2b (c.442C>T), or codon 155 of PITX2c (c.463C>T), predicting the transition of glutamine-encoding codon to a stop codon at amino acid 102 for PITX2a (p.Q102X), or 148 for PITX2b (p.Q148X), or 155 for PITX2c (p.Q155X), was identified in an ECD patient with positive family history. The sequence electropherograms showing the identified nonsense PITX2 variation compared with the corresponding control sequence are shown in Fig 1A. The schematic diagrams showing the structural domains of the wild-type and mutant PITX2 proteins are presented in Fig 1B. The variation was neither observed in 800 control chromosomes nor reported in the EVS’s and NCBI’s SNP databases, which were consulted again on September 1, 2014. Genetic screening of the mutation carrier’s family members demonstrated that the variation was present in all affected family members available, but absent in unaffected family members examined. Analysis of the pedigree showed that in the family the mutation co-segregated with ECD transmitted as an autosomal dominant trait with complete penetrance. The pedigree structure of the family is illustrated in Fig 1C. Besides, the proband (III-3) had also transposition of the great arteries, and her father (II-5) and uncle (II-1) had also mitral valve cleft and right aortic arch. Interestingly, all the mutation carriers had also oligodontia, maxillary hypoplasia and iris hypoplasia, and the proband (III-3) and her father (II-5) had also congenital umbilical hernia, a phenotype of Axenfeld-Rieger syndrome (ARS). The phenotypic characteristics and results of genetic screening of the affected pedigree members are listed in Table 3.
Fig 1. PITX2 mutation associated with endocardial cushion defect and Axenfeld-Rieger syndrome.
(A) Sequence electropherograms showing the heterozygous PITX2 mutation compared with its control. The arrow indicates the heterozygous nucleotides of C/T in the proband (mutant) or the homozygous nucleotides of C/C in the corresponding control individual (wild-type). The rectangle signifies the nucleotides comprising a codon of PITX2. (B) Schematic diagrams showing the structural domains of wild-type and mutant PITX2 proteins with the disease related mutation indicated. The mutation found in patients with endocardial cushion defect and Axenfeld-Rieger syndrome is shown above the structural domains of the mutant PITX2 proteins. NH2 denotes amino-terminus; TAD1, transcriptional activation domain 1; HD, homeodomain; NLS, nuclear localization signal; TID1, transcriptional inhibitory domain 1; TAD2, transcriptional activation domain 2; TID2, transcriptional inhibitory domain 2; COOH, carboxyl-terminus. (C) Pedigree structure of the family with endocardial cushion defect and Axenfeld-Rieger syndrome. Family members are identified by generations and numbers. Square indicates male family member; circle, female member; symbol with a slash, the deceased member; closed symbol, affected member; open symbol, unaffected member; arrow, proband; “+”, carrier of the heterozygous mutation; “–”, non-carrier.
Table 3. Phenotypic characteristics and status of PITX2 mutation of the affected pedigree members.
| Subject information | Phenotype | Genotype | |||
|---|---|---|---|---|---|
| Identity | Gender | Age (years) | Cardiac defects | Extracardiac defects | PITX2 mutation |
| I-1 | M | 50 a | ECD | OD, MH, IH | NA |
| II-1 | M | 31 | ECD, RAA, MVC | OD, MH, IH | +/– |
| II-5 | M | 26 | ECD, RAA, MVC | OD, MH, IH, UH | +/– |
| III-3 | F | 1 | ECD, TGA | OD, MH, IH, UH | +/– |
M, male; F, female; ECD, endocardial cushion defect; MVC, mitral valve cleft; RAA, right aortic arch; TGA, transposition of the great arteries; OD, oligodontia; MH, maxillary hypoplasia; IH, iris hypoplasia; UH, umbilical hernia; NA, not available; +/–, heterozygote.
aAge at death.
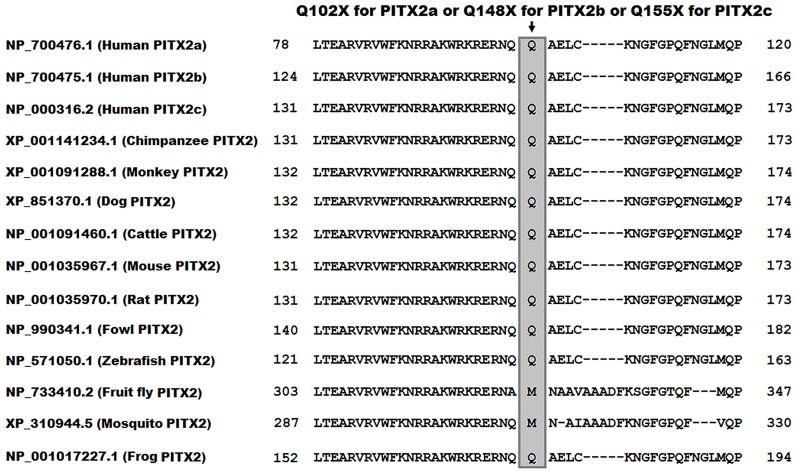
Multiple alignments of PITX2 protein sequences across species
A cross-species alignment of PITX2 protein sequences displayed that the altered amino acid, p.Q102 for PITX2a, or p.Q148 for PITX2b, or p.Q155 for PITX2c, was completely conserved evolutionarily among all vertebrates (Fig 2).
Fig 2. Alignment of multiple PITX2 amino acid sequences among species.
The altered amino acid of p.Q102 for PITX2a, or p.Q148 for PITX2b, or p.Q155 for PITX2c is completely conserved evolutionarily among vertebrates.
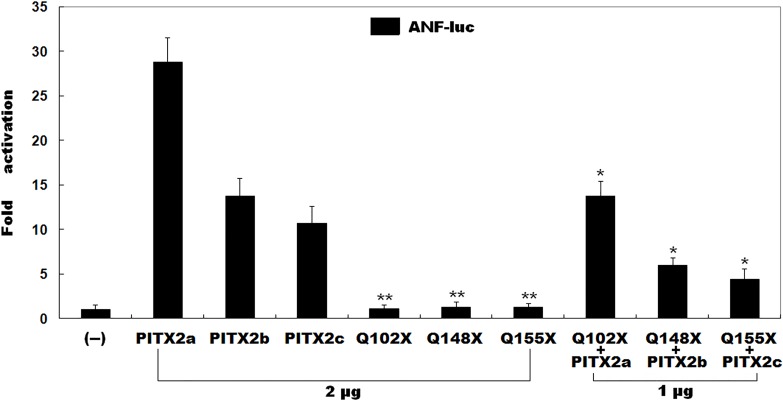
Transactivational activity of the mutant PITX2
As shown in Fig 3, the wild-type PITX2a, PITX2b and PITX2c activated the ANF promoter by ~29-fold, ~14-fold and ~11-fold, respectively; whereas the same amount (2 μg) of mutant PITX2a, PITX2b or PITX2c activated the ANF promoter by ~1-fold. When the same amount of wild-type PITX2 (1 μg) was cotransfected with mutant PITX2 (1 μg), the induced activation of the ANF promoter was ~14-fold for PITX2a, ~6-fold for PITX2b and ~4-fold for PITX2c. These results suggest that the mutant PITX2 has no transactivational activity when compared with its wild-type counterpart.
Fig 3. Transactivational defects caused by PITX2 mutation.
Transcriptional activation of atrial natriuretic factor promoter driven luciferase reporter in CHO cells by wild-type or mutant PITX2, alone or in combination, showed that the mutant PITX2 did not transactivate gene expression. Data are derived from three independent experiments repeated in triplicate. Mean fold activation and standard deviations are shown. ** and * represent P<0.001 and P<0.01, respectively, when compared with wild-type PITX2.
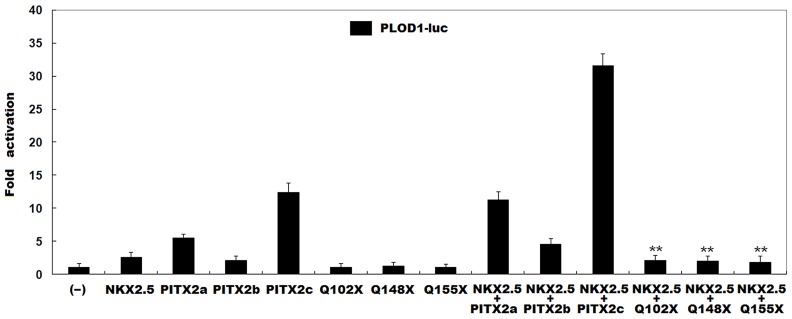
Synergistic transcriptional activity between mutant PITX2 and NKX2.5
As shown in Fig 4, in the presence of 2μg of wild-type NKX2.5, 2μg of wild-type PITX2a, PITX2b and PITX2c activated the PLOD1 promoter by ~11-fold, ~5-fold and ~32-fold, respectively; while the same amount (2μg) of Q102X-mutant PITX2a, or Q148-mutant PITX2b or Q155X-mutant PITX2c activated the PLOD1 promoter by ~2-fold, indicating that the mutation blocks the synergistic transactivational activity between PITX2 and NKX2.5.
Fig 4. No synergistic transcriptional activation between NKX2.5 and mutant PITX2.
The synergistic transactivation of the PLOD1 promoter in CHO cells by NKX2.5 and mutant PITX2 was eliminated by the mutation. All data are derived from three independent experiments repeated in triplicate. Mean fold activation and standard deviations are shown. ** represents P<0.001, when compared with NKX2.5 plus wild-type PITX2.
Discussion
In the current study, a novel heterozygous mutation in the PITX2 gene, p.Q102X for PITX2a, p.Q148X for PITX2b, or p.Q155X for PITX2c, was identified in a family with congenital ECD and ARS. Genetic analysis of the pedigree showed that the nonsense mutation was transmitted in an autosomal dominant pattern with complete penetrance. The mutation, which was absent in the 800 reference chromosomes, altered the amino acid highly conserved evolutionarily among vertebrates. Functional assays unveiled that each isoform of the mutant PITX2 lost the ability to transactivate the ANF and PLOD1 promoters and that the mutation eliminated the synergistic transcriptional activation between PITX2 and NKX2.5. Hence, it is very likely that genetically defective PITX2 confers enhanced susceptibility to ECD and ARS in these mutation carriers.
It has been revealed that PITX2 is abundantly expressed in the developing hearts, craniofacial organs, and abdominal wall, especially in myocardium related to endocardial cushions of the atrioventricular canal, and functions to mediate multiple target genes that are amply expressed during embryogenesis, including ANF and PLOD1 [66–74]. Therefore, the transcriptional effect of a mutant PITX2 may be characterized by using the ANF and PLOD1 promoters. In this study, functional analyses demonstrated that the mutation identified in patients with ECD and ARS abolished the transcriptional activation of ANF- or PLOD1-driven luciferase reporter by PITX2 and eliminated the transcriptionally synergistic activation between PITX2 and NKX2.5, indicating that functionally impaired PITX2 is potentially an alternative molecular mechanism underpinning CHD and ARS.
Previous studies have established that multiple important genes are transcriptionally regulated by PITX2c during cardiovascular development [87], and mutations in several target genes, such as NKX2.5 and GATA4, have been causally implicated in CHD including ECD [40–48,51–58]. Therefore, mutated PITX2c may increase the vulnerability to CHD by altering the expressions of such cardiac-specific target genes.
In humans, PITX2c mutations have been implicated in the pathogenesis of other CHDs. Wang and co-workers [82] screened PITX2c in 382 unrelated patients with CHDs and found two heterozygous mutations, p.W147X and p.N153D, in two patients with CHD, respectively, including a one-year-old male patient with double outlet right ventricle in combination with ventricular septal defect and a four-year-old female patient with isolated ventricular septal defect. Yuan et al. [83] scanned PITX2c in 150 unrelated patients with CHDs and identified two novel heterozygous PITX2c mutations, p.H98Q and p.M119T, in two patients with atrial septal defects, respectively. Wei and colleagues [84] also sequenced PITX2c in 170 unrelated neonates with CHDs and detected two novel heterozygous PITX2c mutations, p.R91Q and p.T129S, in two unrelated newborns with transposition of the great arteries and ventricular septal defect, respectively. Functional analysis demonstrated that all the above-mentioned PITX2c mutations were consistently associated with significantly diminished transcriptional activity [82–84]. In this study, a novel PITX2 loss-of-function mutation is identified in patients with ECD and ARS, thus expanding the phenotypic spectrum linked to PITX2 mutation.
Association of genetically compromised PITX2 with enhanced susceptibility to ECD has been demonstrated in animal models [79–81]. In mice, PITX2 deficiency results in complicated cardiac defects, including atrial septal defect, ventricular septal defect, ECD, hypoplasia of the right ventricle, and failure to form normal cardiac valves [81]. Further studies shows that ablation of PITX2 results in distortion, rather than loss, of muscle anlagen, suggesting that its function becomes critical during the colonization of, and/or fiber assembly in, the anlagen. In addition, myogenic cells lacking PITX2 are smaller and more symmetrical with decreased motility, which may prevent proper assembly of higher-order fibers within anlagen [88]. Nevertheless, PITX2c expression in mesenchymal cushion cells remains a controversial topic. Furtado and colleagues [70] reported that in mice PITX2c was expressed in trabecular and septal, as well as non-trabecular, myocardium, and had a strong expression bias in myocardium associated with individual endocardial cushions of the atrioventricular canal and outflow tract, which are essential for cardiac septation. Two other groups [80,89] also reported the expression of PITX2c in these structures. Fate-mapping studies using a PITX2 cre recombinase knock-in allele showed that daughters of PITX2-expressing cells populated the right and left ventricles, atrioventricular cushions and valves and pulmonary veins. In PITX2 mutant embryos, descendents of PITX2-expressing cells failed to contribute to the atrioventricular cushions and valves and the pulmonary vein, resulting in abnormal morphogenesis of these structures [80]. However, lineage-tracing studies in mice showed that myocardium did not transform into mesenchyme in cushions [90]. In humans, PITX2c was expressed predominantly in left atria, with lower levels in right atrium and left and right ventricles [72]. Due to pronounced spatial and temporal difference in gene expression even for the same species, further work will be necessary to clarify this issue, especially for all isoforms of PITX2 in human heart.
Up to now, in humans mutated PITX2 has been linked to type 1 ARS [66–68], type 2 iridogoniodysgenesis [91], Peters’ anomaly [92], ring dermoid of cornea [93], various congenital heart diseases [16,82–84], and atrial fibrillation [94–97]. In this study, a novel PITX2 mutation was linked to atypical ARS with ECD being the main phenotype. The remarkable phenotypic diversities caused by PITX2 mutations may be explained as follows. Firstly, different genetic backgrounds, including possibly common SNPs altering disease susceptibility, contribute to the variable phenotypes. Secondly, distinct epigenetic modifiers may account for the significant phenotypic heterogeneity among these mutation carriers. Thirdly, delayed penetrance or incomplete penetrance may also be responsible for the discrepant clinical expressivity. Finally, mutations as found in this study may be merely a genetic risk factor predisposing to a disease, rather than a direct cause, and environmental risk factors may be required for the onset of the disease [98].
Conclusions
In conclusion, this study firstly links PITX2 loss-of-function mutation to ECD and ARS, which provides novel insight into the molecular mechanisms of CHD and ARS, implying potential implications in antenatal prophylaxis and personalized treatment of CHD and ARS.
Acknowledgments
The authors are thankful to the participants for their participation in the study.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants from the National Natural Science Fund of China (81270161, 81270231, and 81370298), the National Basic Research Program of China (2012CB9668003), and the key basic research program of Shanghai, China (14JC1405500).
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2014) Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129: e28–e292. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Müller J, Hess J, Hager A (2012) Minor symptoms of depression in patients with congenital heart disease have a larger impact on quality of life than limited exercise capacity. Int J Cardiol 154: 265–269. 10.1016/j.ijcard.2010.09.029 [DOI] [PubMed] [Google Scholar]
- 3. Mulkey SB, Swearingen CJ, Melguizo MS, Schmitz ML, Ou X, Ramakrishnaiah RH, et al. (2013) Multi-tiered analysis of brain injury in neonates with congenital heart disease. PediatrCardiol 34: 1772–1784. 10.1007/s00246-013-0712-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann A, Chockalingam P, Balint OH, Dadashev A, Dimopoulos K, Engel R, et al. (2010) Cerebrovascular accidents in adult patients with congenital heart disease. Heart 96: 1223–1226. 10.1136/hrt.2010.196147 [DOI] [PubMed] [Google Scholar]
- 5. Dimopoulos K, Wort SJ, Gatzoulis MA (2014) Pulmonary hypertension related to congenital heart disease: a call for action. Eur Heart J 35: 691–700. 10.1093/eurheartj/eht437 [DOI] [PubMed] [Google Scholar]
- 6. Alonso-Gonzalez R, Borgia F, Diller GP, Inuzuka R, Kempny A, Martinez-Naharro A, et al. (2013) Abnormal lung function in adults with congenital heart disease: prevalence, relation to cardiac anatomy, and association with survival. Circulation 127: 882–890. 10.1161/CIRCULATIONAHA.112.126755 [DOI] [PubMed] [Google Scholar]
- 7. Martínez-Quintana E, Rodríguez-González F, Nieto-Lago V (2013) Subclinical hypothyroidism in grown-up congenital heart disease patients. PediatrCardiol 34: 912–917. 10.1007/s00246-012-0571-6 [DOI] [PubMed] [Google Scholar]
- 8. Ohuchi H, Miyamoto Y, Yamamoto M, Ishihara H, Takata H, Miyazaki A, et al. (2009) High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J 158: 30–39. 10.1016/j.ahj.2009.04.021 [DOI] [PubMed] [Google Scholar]
- 9. Kröönström LA, Johansson L, Zetterström AK, Dellborg M, Eriksson P, Cider Å (2014) Muscle function in adults with congenital heart disease.Int J Cardiol 170: 358–363. 10.1016/j.ijcard.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 10. Moutafi AC, Manis G, Dellos C, Tousoulis D, Davos CH(2014) Cardiac autonomic nervous activity in adults with coarctation of the aorta late after repair. Int J Cardiol 173: 566–568. 10.1016/j.ijcard.2014.03.120 [DOI] [PubMed] [Google Scholar]
- 11. Rushani D, Kaufman JS, Ionescu-Ittu R, Mackie AS, Pilote L, Therrien J, et al. (2013) Infective endocarditis in children with congenital heart disease: cumulative incidence and predictors. Circulation 128: 1412–1419. 10.1161/CIRCULATIONAHA.113.001827 [DOI] [PubMed] [Google Scholar]
- 12. Fahed AC, Roberts AE, Mital S, Lakdawala NK (2014) Heart failure in congenital heart disease: a confluence of acquired and congenital. Heart Fail Clin 10: 219–227. 10.1016/j.hfc.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perry JC (2012) Sudden cardiac death and malignant arrhythmias: the scope of the problem in adult congenital heart patients. PediatrCardiol 33: 484–490. 10.1007/s00246-012-0171-5 [DOI] [PubMed] [Google Scholar]
- 14. Tutarel O, Kempny A, Alonso-Gonzalez R, Jabbour R, Li W, Uebing A, et al. (2014) Congenital heart disease beyond the age of 60: emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur Heart J 35: 725–732. 10.1093/eurheartj/eht257 [DOI] [PubMed] [Google Scholar]
- 15. Hunter RM, Isaac M, Frigiola A, Blundell D, Brown K, Bull K (2013) Lifetime costs and outcomes of repair of Tetralogy of Fallot compared to natural progression of the disease: Great Ormond Street Hospital cohort. PLoS One 8: e59734 10.1371/journal.pone.0059734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, et al. (2013) De novo mutations in histone-modifying genes in congenital heart disease. Nature 498: 220–223. 10.1038/nature12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang W, Wang Y, Gong F, Zhu W, Fu S (2013) MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case-control and TDT studies. PLoS One 8: e58041 10.1371/journal.pone.0058041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C, Xie L, Zhou K, Zhan Y, Li Y, Li H, et al. (2013) Increased risk for congenital heart defects in children carrying the ABCB1 Gene C3435T polymorphism and maternal periconceptional toxicants exposure. PLoS One 8: e68807 10.1371/journal.pone.0068807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang SW, Mislankar M, Misra C, Huang N, Dajusta DG, Harrison SM, et al. (2013) Genetic abnormalities in FOXP1 are associated with congenital heart defects. Hum Mutat 34: 1226–1230. 10.1002/humu.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanchez-Castro M, Gordon CT, Petit F, Nord AS, Callier P, Andrieux J, et al. (2013) Congenital heart defects in patients with deletions upstream of SOX9. Hum Mutat 34: 1628–1631. 10.1002/humu.22449 [DOI] [PubMed] [Google Scholar]
- 21. Lukasz A, Beutel G, Kümpers P, Denecke A, Westhoff-Bleck M, Schieffer B, et al. (2013) Angiopoietin-2 in adults with congenital heart disease and heart failure. PLoS One 8: e66861 10.1371/journal.pone.0066861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Long F, Wang X, Fang S, Xu Y, Sun K, Chen S, et al. (2013) A potential relationship among beta-defensins haplotype, SOX7 duplication and cardiac defects. PLoS One 8: e72515 10.1371/journal.pone.0072515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lahm H, Deutsch MA, Dreßen M, Doppler S, Werner A, Hörer J, et al. (2013) Mutational analysis of the human MESP1 gene in patients with congenital heart disease reveals a highly variable sequence in exon 1. Eur J Med Genet 56: 591–598. 10.1016/j.ejmg.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 24. Wang E, Jin W, Duan W, Qiao B, Sun S, Huang G, et al. (2013) Association of two variants in SMAD7 with the risk of congenital heart disease in the Han Chinese population.PLoS One 8: e72423 10.1371/journal.pone.0072423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen D, Qiao Y, Meng H, Pang S, Huang W, Zhang H, et al. (2013) Genetic analysis of the TBX3 gene promoter in ventricular septal defects. Gene 512: 185–188. 10.1016/j.gene.2012.10.066 [DOI] [PubMed] [Google Scholar]
- 26. Wu M, Li Y, He X, Shao X, Yang F, Zhao M, et al. (2013) Mutational and functional analysis of the BVES gene coding region in Chinese patients with non-syndromic tetralogy of Fallot. Int J Mol Med 31: 899–903. 10.3892/ijmm.2013.1275 [DOI] [PubMed] [Google Scholar]
- 27. Gong X, Wu X, Ma X, Wu D, Zhang T, He L, et al. (2013) Microdeletion and microduplication analysis of chineseconotruncal defects patients with targeted array comparative genomic hybridization. PLoS One 8: e76314 10.1371/journal.pone.0076314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al Turki S, Manickaraj AK, Mercer CL, Gerety SS, Hitz MP, Lindsay S, et al. (2014) Rare variants in NR2F2 cause congenital heart defects in humans. Am J Hum Genet 94: 574–585. 10.1016/j.ajhg.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bansal V, Dorn C, Grunert M, Klaassen S, Hetzer R, Berger F, et al. (2014) Outlier-based identification of copy number variations using targeted resequencing in a small cohort of patients with Tetralogy of Fallot. PLoS One 9: e85375 10.1371/journal.pone.0085375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu X, Deng X, Huang G, Wang J, Yang J, Chen S, et al. (2014) A novel mutation of Hyaluronan synthase 2 gene in Chinese children with ventricular septal defect. PLoS One 9: e87437 10.1371/journal.pone.0087437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu AP, Chow PC, Lee PP, Mok GT, Tang WF, Lau ET, et al. (2014) Under-recognition of 22q11.2 deletion in adult Chinese patients with conotruncal anomalies: implications in transitional care. Eur J Med Genet 57: 306–311. 10.1016/j.ejmg.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 32. Lin B, Wang Y, Wang Z, Tan H, Kong X, Shu Y, et al. (2014) Uncovering the rare variants of DLC1 isoform 1 and their functional effects in a Chinese sporadic congenital heart disease cohort. PLoS One 9: e90215 10.1371/journal.pone.0090215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu J, Lin Y, Si L, Jin G, Dai J, Wang C, et al. (2014) Genetic variants at 10p11 confer risk of Tetralogy of Fallot in Chinese of Nanjing. PLoS One 9: e89636 10.1371/journal.pone.0089636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cowan J, Tariq M, Ware SM (2014) Genetic and functional analyses of ZIC3 variants in congenital heart disease. Hum Mutat 35: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai B, Zhang T, Zhong R, Zou L, Zhu B, Chen W, et al. (2014) Genetic variant in MTRR, but not MTR, is associated with risk of congenital heart disease: an integrated meta-analysis. PLoS One 9: e89609 10.1371/journal.pone.0089609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Y, Wang F, Wu Y, Tan S, Wen Q, Wang J, et al. (2014) Variations of CITED2 are associated with congenital heart disease (CHD) in Chinese population. PLoS One 9: e98157 10.1371/journal.pone.0098157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lozić B, Krželj V, Kuzmić-Prusac I, Kuzmanić-Šamija R, Capkun V, Lasan R, et al. (2014) The OSR1 rs12329305 Polymorphism Contributes to the Development of Congenital Malformations in Cases of Stillborn/Neonatal Death. Med SciMonit 20: 1531–1538. 10.12659/MSM.890916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lalani SR, Belmont JW (2014) Genetic basis of congenital cardiovascular malformations. Eur J Med Genet 57: 402–413. 10.1016/j.ejmg.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Töpf A, Griffin HR, Glen E, Soemedi R, Brown DL, Hall D, et al. (2014) Functionally significant, rare transcription factor variants in tetralogy of fallot. PLoS One 9: e95453 10.1371/journal.pone.0095453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, et al. (1998) Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 281: 108–111. [DOI] [PubMed] [Google Scholar]
- 41. Reamon-Buettner SM, Borlak J (2010) NKX2-5: an update on this hypermutable homeodomain protein and its role in human congenital heart disease (CHD). Hum Mutat 31: 1185–1194. 10.1002/humu.21345 [DOI] [PubMed] [Google Scholar]
- 42. Huang W, Meng H, Qiao Y, Pang S, Chen D, Yan B (2013) Two novel and functional DNA sequence variants within an upstream enhancer of the human NKX2-5 gene in ventricular septal defects. Gene 524: 152–155. 10.1016/j.gene.2013.04.043 [DOI] [PubMed] [Google Scholar]
- 43. Wang Z, Zou L, Zhong R, Zhu B, Chen W, Shen N, et al. (2013) Associations between two genetic variants in NKX2-5 and risk of congenital heart disease in Chinese population: a meta-analysis. PLoS One 8: e70979 10.1371/journal.pone.0070979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reamon-Buettner SM, Sattlegger E, Ciribilli Y, Inga A, Wessel A, Borlak J (2013) Transcriptional defect of an inherited NKX2-5 haplotype comprising a SNP, a nonsynonymous and a synonymous mutation, associated with human congenital heart disease. PLoS One 8: e83295 10.1371/journal.pone.0083295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Costa MW, Guo G, Wolstein O, Vale M, Castro ML, Wang L, et al. (2013) Functional characterization of a novel mutation in NKX2-5 associated with congenital heart disease and adult-onset cardiomyopathy. CircCardiovasc Genet 6: 238–247. 10.1161/CIRCGENETICS.113.000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang RT, Xue S, Xu YJ, Zhou M, Yang YQ (2013) A novel NKX2.5 loss-of-function mutation responsible for familial atrial fibrillation. Int J Mol Med 31: 1119–1126. 10.3892/ijmm.2013.1316 [DOI] [PubMed] [Google Scholar]
- 47. Carter DR, Buckle AD, Tanaka K, Perdomo J, Chong BH (2014) Art27 interacts with GATA4, FOG2 and NKX2.5 and is a novel co-repressor of cardiac genes. PLoS One 9: e95253 10.1371/journal.pone.0095253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kobayashi J, Yoshida M, Tarui S, Hirata M, Nagai Y, Kasahara S, et al. (2014) Directed differentiation of patient-specific induced pluripotent stem cells identifies the transcriptional repression and epigenetic modification of NKX2-5, HAND1, and NOTCH1 in hypoplastic left heart syndrome. PLoS One 9: e102796 10.1371/journal.pone.0102796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao L, Ni SH, Liu XY, Wei D, Yuan F, Xu L, et al. (2014) Prevalence and spectrum of Nkx2.6 mutations in patients with congenital heart disease. Eur J Med Genet 57: 579–586. 10.1016/j.ejmg.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Zhang DF, Sun YM, Li RG, Qiu XB, Qu XK, et al. (2014) NKX2-6 mutation predisposes to familial atrial fibrillation. Int J Mol Med 34: 1581–1590. 10.3892/ijmm.2014.1971 [DOI] [PubMed] [Google Scholar]
- 51. Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, et al. (2003) GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 424: 443–447. [DOI] [PubMed] [Google Scholar]
- 52. Rajagopal SK, Ma Q, Obler D, Shen J, Manichaikul A, Tomita-Mitchell A, et al. (2007) Spectrum of heart disease associated with murine and human GATA4 mutation. J Mol Cell Cardiol 43: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang YQ, Gharibeh L, Li RG, Xin YF, Wang J, Liu ZM, et al. (2013) GATA4 loss-of-function mutations underlie familial tetralogy of fallot. Hum Mutat 34: 1662–1671. 10.1002/humu.22434 [DOI] [PubMed] [Google Scholar]
- 54. Wang E, Sun S, Qiao B, Duan W, Huang G, An Y, et al. (2013) Identification of functional mutations in GATA4 in patients with congenital heart disease. PLoS One 8: e62138 10.1371/journal.pone.0062138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang YQ, Wang J, Liu XY, Chen XZ, Zhang W, Wang XZ (2013) Mutation spectrum of GATA4 associated with congenital atrial septal defects. Arch Med Sci 9: 976–983. 10.5114/aoms.2013.39788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li RG, Li L, Qiu XB, Yuan F, Xu L, Li X, et al. (2013) GATA4 loss-of-function mutation underlies familial dilated cardiomyopathy. BiochemBiophys Res Commun 439: 591–596. 10.1016/j.bbrc.2013.09.023 [DOI] [PubMed] [Google Scholar]
- 57. Xiang R, Fan LL, Huang H, Cao BB, Li XP, Peng DQ, et al. (2014) A novel mutation of GATA4 (K319E) is responsible for familial atrial septal defect and pulmonary valve stenosis. Gene 534: 320–323. [PubMed] [Google Scholar]
- 58. Li J, Liu WD, Yang ZL, Yuan F, Xu L, Li RG, et al. (2014) Prevalence and spectrum of GATA4 mutations associated with sporadic dilated cardiomyopathy. Gene 548: 174–181. 10.1016/j.gene.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 59. Jiang JQ, Li RG, Wang J, Liu XY, Xu YJ, Fang WY, et al. (2013) Prevalence and spectrum of GATA5 mutations associated with congenital heart disease. Int J Cardiol 165: 570–573. 10.1016/j.ijcard.2012.09.039 [DOI] [PubMed] [Google Scholar]
- 60. Huang RT, Xue S, Xu YJ, Zhou M, Yang YQ (2014) Somatic GATA5 mutations in sporadic tetralogy of Fallot. Int J Mol Med 33: 1227–1235. 10.3892/ijmm.2014.1674 [DOI] [PubMed] [Google Scholar]
- 61. Shi LM, Tao JW, Qiu XB, Wang J, Yuan F, Xu L, et al. (2014) GATA5 loss-of-function mutations associated with congenital bicuspid aortic valve. Int J Mol Med 33: 1219–1226. 10.3892/ijmm.2014.1700 [DOI] [PubMed] [Google Scholar]
- 62. Huang RT, Xue S, Xu YJ, Yang YQ (2013) Somatic mutations in the GATA6 gene underlie sporadic tetralogy of Fallot. Int J Mol Med 31: 51–58. 10.3892/ijmm.2012.1188 [DOI] [PubMed] [Google Scholar]
- 63. Xu L, Zhao L, Yuan F, Jiang WF, Liu H, Li RG, et al. (2014) GATA6 loss-of-function mutations contribute to familial dilated cardiomyopathy. Int J Mol Med 34: 1315–1322. 10.3892/ijmm.2014.1896 [DOI] [PubMed] [Google Scholar]
- 64. Liang D, Zhen L, Yuan T, Huang J, Deng F, Wu YH, et al. (2014) miR-10a regulates proliferation of human cardiomyocyte progenitor cells by targeting GATA6.PLoS One 9: e103097 10.1371/journal.pone.0103097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McCulley DJ, Black BL (2012) Transcription factor pathways and congenital heart disease. Curr Top Dev Biol 100: 253–277. 10.1016/B978-0-12-387786-4.00008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, et al. (1996) Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet 14: 392–399. [DOI] [PubMed] [Google Scholar]
- 67. Hjalt TA, Semina EV (2005) Current molecular understanding of Axenfeld-Rieger syndrome. Expert Rev Mol Med 7: 1–17. [DOI] [PubMed] [Google Scholar]
- 68. Tumer Z, Bach-Holm D (2009) Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet 17: 1527–1539. 10.1038/ejhg.2009.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Simard A, Di Giorgio L, Amen M, Westwood A, Amendt BA, Ryan AK (2009) The Pitx2c N-terminal domain is a critical interaction domain required for asymmetric morphogenesis. Dev Dyn 238: 2459–2470. 10.1002/dvdy.22062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Furtado MB, Biben C, Shiratori H, Hamada H, Harvey RP (2011) Characterization of Pitx2c expression in the mouse heart using a reporter transgene. Dev Dyn 240:195–203. 10.1002/dvdy.22492 [DOI] [PubMed] [Google Scholar]
- 71. Kahr PC, Piccini I, Fabritz L, Greber B, Scholer H, Scheld HH, et al. (2011) Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. PLoSOne 6: e26389 10.1371/journal.pone.0026389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kirchhof P, Kahr PC, Kaese S, Piccini I, Vokshi I, Scheld HH, et al. (2011) PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. CircCardiovasc Genet 4: 123–133. 10.1161/CIRCGENETICS.110.958058 [DOI] [PubMed] [Google Scholar]
- 73. Hsu J, Hanna P, Van Wagoner DR, Barnard J, Serre D, Chung MK, et al. (2012) Whole genome expression differences in human left and right atria ascertained by RNA sequencing. CircCardiovasc Genet 5: 327–335. 10.1161/CIRCGENETICS.111.961631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Torrado M, Franco D, Hernández-Torres F, Crespo-Leiro MG, Iglesias-Gil C, Castro-Beiras A, et al. (2014) Pitx2c is reactivated in the failing myocardium and stimulates myf5 expression in cultured cardiomyocytes. PLoS One 9: e90561 10.1371/journal.pone.0090561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schweickert A, Campione1 M, Steinbeisser H, Blum M (2000) Pitx2 isoforms: involvement of Pitx2c but not Pitx2a or Pitx2b in vertebrate left-right asymmetry. Mech Dev 90: 41–51. [DOI] [PubMed] [Google Scholar]
- 76. Liu C, Liu W, Lu MF, Brown NA, Martin JF (2001) Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development 128: 2039–2048. [DOI] [PubMed] [Google Scholar]
- 77. Galli D, Domínguez JN, Zaffran S, Munk A, Brown NA, Buckingham ME (2008) Atrial myocardium derives from the posterior region of the second heart field, which acquires left-right identity as Pitx2c is expressed. Development 135: 1157–1167. 10.1242/dev.014563 [DOI] [PubMed] [Google Scholar]
- 78. Lozano-Velasco E, Chinchilla A, Martínez-Fernández S, Hernández-Torres F, Navarro F, Lyons GE, et al. (2011) Pitx2c modulates cardiac-specific transcription factors networks in differentiating cardiomyocytes from murine embryonic stem cells. Cells Tissues Organs 194: 349–362. 10.1159/000323533 [DOI] [PubMed] [Google Scholar]
- 79. Dagle JM, Sabel JL, Littig JL, Sutherland LB, Kolker SJ, Weeks DL (2003) Pitx2c attenuation results in cardiac defects and abnormalities of intestinal orientation in developing Xenopuslaevis. Dev Biol 262: 268–281. [DOI] [PubMed] [Google Scholar]
- 80. Liu C, Liu W, Palie J, Lu MF, Brown NA, Martin JF (2002) Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development 129: 5081–5091. [DOI] [PubMed] [Google Scholar]
- 81. Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, et al. (1999) Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 126: 5749–5758. [DOI] [PubMed] [Google Scholar]
- 82. Wang J, Xin YF, Xu WJ, Liu ZM, Qiu XB, Qu XK, et al. (2013) Prevalence and spectrum of PITX2c mutations associated with congenital heart disease. DNA Cell Biol 32: 708–716. 10.1089/dna.2013.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yuan F, Zhao L, Wang J, Zhang W, Li X, Qiu XB, et al. (2013) PITX2c loss-of-function mutations responsible for congenital atrial septal defects. Int J Med Sci 10: 1422–1429. 10.7150/ijms.6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wei D, Gong XH, Qiu G, Wang J, Yang YQ (2014) Novel PITX2c loss-of-function mutations associated with complex congenital heart disease. Int J Mol Med 33: 1201–1208. 10.3892/ijmm.2014.1689 [DOI] [PubMed] [Google Scholar]
- 85. Hjalt TA, Amendt BA, Murray JC (2001) PITX2 regulates procollagen lysyl hydroxylase (PLOD) gene expression: implications for the pathology of Rieger syndrome. J Cell Biol 152: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cox CJ, Espinoza HM, McWilliams B, Chappell K, Morton L, Hjalt TA, et al. (2002) Differential regulation of gene expression by PITX2 isoforms. J BiolChem 277: 25001–25010. [DOI] [PubMed] [Google Scholar]
- 87. Ganga M, Espinoza HM, Cox CJ, Morton L, Hjalt TA, Lee Y, et al. (2003) PITX2 isoform-specific regulation of atrial natriuretic factor expression: synergism and repression with Nkx2.5. J BiolChem 278: 22437–22445. [DOI] [PubMed] [Google Scholar]
- 88. Campbell AL, Shih HP, Xu J, Gross MK, Kioussi C (2012) Regulation of motility of myogenic cells in filling limb muscle anlagen by Pitx2. PLoS One 7:e35822 10.1371/journal.pone.0035822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ai D, Liu W, Ma L, Dong F, Lu MF, Wang D, et al. (2006) Pitx2 regulates cardiac left-right asymmetry by patterning second cardiac lineage-derived myocardium. Dev Biol 296:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. de Lange FJ, Moorman AF, Anderson RH, Männer J, Soufan AT, de Gier-de Vries C, et al. (2004) Lineage and morphogenetic analysis of the cardiac valves.Circ Res 95: 645–654. [DOI] [PubMed] [Google Scholar]
- 91. Banerjee-Basu S, Baxevanis AD (1999) Threading analysis of the Pitx2 homeodomain: predicted structural effects of mutations causing Rieger syndrome and iridogoniodysgenesis. Hum Mutat 14: 312–319. [DOI] [PubMed] [Google Scholar]
- 92. Doward W, Perveen R, Lloyd IC, Ridgway AE, Wilson L, Black GC (1999) A mutation in the RIEG1 gene associated with Peters’ anomaly. J Med Genet 36: 152–155. [PMC free article] [PubMed] [Google Scholar]
- 93. Xia K, Wu L, Liu X, Xi X, Liang D, Zheng D, et al. (2004) Mutation in PITX2 is associated with ring dermoid of the cornea. J Med Gene 41: e129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yang YQ, Xu YJ, Li RG, Qu XK, Fang WY, Liu X (2013) Prevalence and spectrum of PITX2c mutations associated with familial atrial fibrillation. Int J Cardiol 168: 2873–2876. 10.1016/j.ijcard.2013.03.141 [DOI] [PubMed] [Google Scholar]
- 95. Zhou YM, Zheng PX, Yang YQ, Ge ZM, Kang WQ (2013) A novel PITX2c loss-of-function mutation underlies lone atrial fibrillation. Int J Mol Med 32: 827–834. 10.3892/ijmm.2013.1463 [DOI] [PubMed] [Google Scholar]
- 96. Wang J, Zhang DF, Sun YM, Yang YQ (2014) A novel PITX2c loss-of-function mutation associated with familial atrial fibrillation. Eur J Med Genet 57: 25–31. 10.1016/j.ejmg.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 97. Qiu XB, Xu YJ, Li RG, Xu L, Liu X, Fang WY, et al. (2014) PITX2C loss-of-function mutations responsible for idiopathic atrial fibrillation. Clinics (Sao Paulo) 69: 15–22. 10.6061/clinics/2014(01)03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yu H, Xu JH, Song HM, Zhao L, Xu WJ, Wang J, et al. (2014) Mutational spectrum of the NKX2-5 gene in patients with lone atrial fibrillation. Int J Med Sci 11: 554–563. 10.7150/ijms.8407 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.