Abstract
Objective
To assess the association between diabetes mellitus (DM) and the incidence and disease-specific mortality of endometrial cancer (EC).
Methods
MEDLINE, EMBASE and conference abstracts of the 2011–2013 Annual Meetings of Society of Gynecological Oncology were searched for reports of original cohort studies that enrolled diabetic and non-diabetic women who were free of EC at baseline to compare the incidence and disease-specific mortality of EC by DM status. The included reports were examined for demographic characteristics of study populations, study design, effect measures and risk of bias. Statistical heterogeneity was evaluated with Chi-square test of the Cochrane Q statistics at the 0.05 significance level and I2 statistic. Publication bias was assessed by visual examination of a funnel plot and the Egger’s test for small-study effects.
Results
Twenty-nine cohort studies (17 prospective, 12 retrospective) were eligible for this review, 23 of which reported EC incidence, five reported disease-specific mortality and one reported both. For incidence of EC among women with versus without DM, the summary relative risk (RR) was 1.89 (95%CI, 1.46–2.45; p < 0.001) and the summary incidence rate ratio was 1.61 (95%CI, 1.51–1.71; p < 0.001). The pooled RR of disease-specific mortality was 1.32 (95%CI, 1.10–1.60; p = 0.003), while results in the studies reporting standardized mortality ratios were inconsistent. There remains considerable amount of clinical and methodological heterogeneity among the included studies; moreover, the hazard ratios for incident EC showed significant statistical heterogeneity and therefore were not quantitatively synthesized.
Conclusions
There is consistent evidence for an independent association between DM and an increased risk of incident EC, while the association between DM and EC-specific mortality remains uncertain. Further studies with better considerations for selection bias, information bias and confounding will further facilitate causal inference involving DM and EC.
Keywords: Diabetes mellitus, Endometrial cancer, Incidence, Disease-specific mortality, Systematic review, Meta-analysis
Introduction
Diabetes mellitus (DM) is a growing pandemic with serious health and economic implications across the world. In 2013, an estimated 382 million people were living with DM, of whom 24.4 million were in the United States (US) and an estimated 5.1 million deaths worldwide were attributed to DM [1]. It is estimated that in 2013, more than 548 billion US dollars were spent on diabetes worldwide and an average of 9800 US dollars was spent for the care of each diabetic patient in the US [1]. Further, because of life-style changes, improved detection and treatment of diabetes and increase in life expectancy, the number of people living with diabetes worldwide will continue to increase, which is projected to be 439 million in 2030, implicating a 54% rise compared to the estimate in 2010 [2].
DM is a well known cause of cardiovascular complications, including myocardial infarction, stroke, renal disease, retinopathy, peripheral vascular disease, and neuropathy. More recently, DM has been shown to be associated with an increase in cancer risk by epidemiologic and mechanistic studies [3]. Observational studies have demonstrated increased risks of cancers of the pancreas [4], colon and rectum [5], esophagus [6], stomach [7], liver and bile ducts [8,9], urinary tract [10], breast [11], and the endometrium [12–16] among patients with DM. Moreover, DM and increased serum glucose concentration have been associated with a rise in cancer-related mortality compared to the general population [17–19]. Mechanistically, insulin resistance and resultant hyperinsulinemia, hyperglycemia, and inflammation have all been proposed to promote carcinogenesis, malignant proliferation and metastasis through insulin-like growth factor-1 (IGF-1) and other biologic pathways [3].
Endometrial cancer is an important cause of morbidity and mortality among women. In addition to the well-described clinical risk factors for endometrial cancer such as age and estrogen use, several cohort studies and recent meta-analyses have shown an association between pre-existing DM and risk of development of endometrial cancer (EC) [12–16].
DM and EC share common risk factors and are both associated with aging. As global trend of population aging continues, women will live longer after menopause, implying increased likelihood to develop DM and EC. Elucidation of the association between DM and EC will provide important motivation for DM prevention. Further, such information will inform clinical decision making involving screening of EC and management of benign uterine neoplasms in diabetic women.
To our knowledge, there are only two prior systematic reviews that were directly focused on the association between DM and EC, both of which were based on observational studies [14,15]. In this systematic review, we aimed to incorporate the latest evidence and reassess the association between DM and incidence as well as disease-specific mortality of EC. We restricted the review to cohort studies to avoid temporal ambiguity and aimed to address the research question with more rigorous methodologies than those used in previous reviews.
Methods
Search strategy
We searched the electronic databases PubMed and Embase for relevant articles enlisting the help of an experienced librarian to identify and include all the appropriate search, keywords, and controlled vocabulary terms (see Appendix A). We also hand-searched the conference abstracts of the 2011–2013 Annual Meetings of Society of Gynecological Oncology, which were published as supplemental issues of Gynecological Oncology. Finally, we hand searched the reference lists of five most recent systematic reviews and meta-analyses relevant to DM and EC to increase the sensitivity of our search.
Study selection
Prospective, retrospective and case-cohort studies were eligible for inclusion in the present review. We restricted the inclusion criteria to these designs to avoid temporal ambiguity between exposure to DM and onset of EC and to minimize recall bias. Women of all ages who had intact uteri and did not have prior history of EC were eligible for enrolment. There was no specific restriction regarding race, ethnicity, comorbidities or past or current use of medications. Exposures of interest are Type 1 DM (T1DM) and Type 2 DM (T2DM). Pre-diabetic statuses that do not meet the diagnosis criteria of diabetes mellitus and other subtypes of diabetes were not eligible for inclusion. Exposures could be ascertained by self-report, linkage with disease registry, hospital records or insurance claims database. Confirmed diagnosis of incident primary EC of all pathological classifications and deaths in these individuals for which EC was the primary diagnosis were the primary outcomes of interest. Benign or pre-malignant endometrial pathologies were excluded from the analysis. Metastatic cancers in the uterus that originated from other organs or systems were also excluded. Further, only studies that reported original data were eligible. There was no restriction on the length of follow-up. Studies that did not provide these outcome estimates directly but reported data that enabled relevant calculations were included. For articles that reported results from identical patient cohorts with identical objectives, only the one with the greatest coverage of the source population or most direct focus on the association between DM and EC was included. When the outcome estimates were reported at multiple time points during follow-up, those that were measured more than one year after diagnosis of diabetes were extracted to calculate the overall risk estimates.
The reviewers independently screened the titles and abstracts of the retrieved articles for full-text reviews. Each article was screened by two reviewers. The articles were labeled as “Include”, “Exclude” or “Unclear”. All the articles that were categorized as “Include” or “Unclear” were subjected to independent full-text review by two reviewers. Reasons of exclusion were documented in each step of screening and disagreement regarding inclusion/exclusion was addressed by mutual discussions. Input from a third reviewer was solicited if consensus could not be reached. Studies that remained labeled as “Include” at the end were included for review.
Data extraction
All the reviewers contributed to data extraction using the data extraction form (See Appendix B). Two reviewers independently extracted information from each included study. Effect measures that were not numerically reported were calculated based on the data presented in the graphs and tables. Data that could not be computed by this means were considered as missing. The results were compared and discrepancies were addressed by mutual discussions.
Assessment of the included studies
Each included study was critically appraised for methodological quality by two reviewers independently. Appraisal was guided by a Risk of Bias Assessment Form (see Appendix C) adapted from The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [20] and mainly focused on selection bias (similarity between the study population and the source population, informative censoring, etc.), information bias (validity of the information on exposure and outcome, method used to handle missing data, etc.) and confounding (whether the widely-recognized confounders for epidemiologic studies of EC, such as parity, obesity, smoking, oral contraceptives, hormone replacement therapy, age at menarche, polycystic ovary syndrome or other ovulation disorders [21], were controlled for in the study). Every study was rated as “High Risk”, “Low Risk” or “Unclear” for each of these three items. Discrepancies were first reconciled between the pair of reviewers and a third reviewer was consulted when consensus could not be reached. Additionally, clinical and methodological heterogeneity of the included studies were assessed based on characteristics of the study populations, definitions and ascertainment of the exposures and outcomes, lengths of follow-up, measurement and control of confounding factors, and other critical design features
Statistical analysis
A narrative summary of the study results as well as clinical, methodological and statistical heterogeneity was performed before quantitative synthesis was conducted. Because considerable heterogeneity was observed, a random-effect model was used for the meta-analysis [22]. Moreover, because standardized incidence and mortality ratios (SIR and SMR) derived by the indirect age-standardization in different studies are based on different reference populations and are not directly comparable, only relative risk (RR), hazard ratio (HR), incidence rate ratio (IRR) and odds ratio (OR) were considered for quantitative synthesis. Unadjusted effect measures were excluded from quantitative synthesis to minimize confounding.
Statistical heterogeneity for studies reporting the same effect measures was evaluated by Chi-square test of the Cochrane Q statistic at the 0.05 significance level and the I2 statistic. I2 ≥ 50% indicated significant statistical heterogeneity [23]. Potential publication bias was assessed by visual examination of a funnel plot along with the Egger’s test for small-study effects [24,25]. Statistical analyses were performed with Stata version 13.1 (College Station, TX: StataCorp, LLP). Data were entered into this software by independent double entry.
Results
Study selection
The literature search was updated on February 12th, 2014 in PubMed and Embase, yielding 1456 and 3692 articles, respectively. After de-duplication of the resulting 5148 articles, 4223 were screened as outlined above. The search of the conference proceedings of the last three Annual Meetings of the Society of Gynecologic Oncology in 2011–2013 yielded 1121 abstracts, which were also screened. Finally, five additional articles were identified by hand-searching the reference lists of the five most recently published systematic reviews related to DM and EC. Out of the total 5349 records that were screened, 5220 were excluded during the title and abstract screenings. Of the remaining 129 studies that underwent full-text reviews, 100 were excluded. The most common reason for exclusion was ineligible study design (n = 57) (Fig. 1). During the full text review we identified two studies that reported follow-up results of the same cohort during the same period of time [26,27]. However, since the study by Weiderpass et al. focused specifically on EC and breast cancer while the one by Adami et al. studied many different cancer outcomes, we decided to exclude the study by Adami et al. For disease-specific mortality, we excluded studies that enrolled patients with EC at baseline to avoid temporal ambiguity. Data extraction was performed for the 29 remaining studies that met the eligibility criteria [13,27–53].
Fig. 1.
Flowchart of study selection1,2,3. 1. Searched on Feb 12th 2014. 2. The cohort studies by Weiderpass et al. and Adami et al. were derived from the same cohort and since the former study focused on endometrial cancer and breast cancer while the second one compared multiple cancer outcomes, only the study by Weiderpass et al. was retained. 3. SR: systematic review.
Study and participant characteristics
Among the 29 included studies, 17 were prospective cohort and 12 were retrospective cohort designs (Table 1). Thirteen studies did not report the cohort sizes. Among the 16 studies that did, the number of included women ranged from 3047 to 1,790,868. In the included studies, more than 5,302,259 women were followed between the years of 1931 and 2010. The studies were published between the years of 1970 and 2013. Duration of study follow-up ranged from 1 to 45 years and median length of follow-up was 15.5 years. Sixteen studies were conducted in Europe, seven were conducted in the United States, four were conducted in Asia, one was conducted in Canada and one was conducted in New Zealand.
Table 1.
Summary of the included studies (grouped by the reported effect measures)a.
| Author | Designb | Country/region | Time of follow-up | Cohort size
|
Mean age (all) (SD) | DM ascertainment methodc | DM typed | EC ascertainment methodc | No. of EC cases/deaths
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | Non-DM | DM | Non-DM | ||||||||
| HR (7) | |||||||||||
| Chen [28] | R | Taiwan | 2000–2008 | 319,310 | 319,308 | 61.0 (12.4)e | ID | 1& 2 | ID | 520 | 372 |
| Johnson [29] | R | Canada | 1994–2006 | 185,100 | 185,100 | 60.7 (13.5) | HR | 2 | RL | 297 | 212 |
| Lai [31] | P | USA | 1996–2007 | – | – | – | UK | UK | UK | – | – |
| Lai [30] | P | USA | 1995–2006 | 14,710 | 184,881 | –f | SR | 1 & 2 | RL | 184 | 1452 |
| Lambe [32] | R | Sweden | 1985–2002 | 5615 | 225,122 | 46.6 (13.6) | HR | 2 | RL | 47 | 1023 |
| Lo [33] | R | Taiwan | 1996–2002 | 895,434 | 895,434 | 60.5 (13.7) | HR | 2 | RL | 673 | 517 |
| Travier [34] | P | New Zealand | 2000–2004 | 739g | – | – | HbA1c | 1 & 2 | RL | 13 | – |
| IRR (2) | |||||||||||
| Carstensen [35] | P | Denmark | 1995–2009 | – | – | – | RL | 1 & 2 | RL | 601 | 8057 |
| Wotton [36] | R | UK | 1963–2008 | 15,898 | 275,564 | – | HR | 1 & 2 | HR | 35 | 377 |
| RR (7) | |||||||||||
| Anderson [37] | P | USA | 1986–1997 | 1325 | 23,150 | –h | SR | 2 | RL | 34 | 307 |
| Burbos [38] | P | UK | 2006–2009 | 183 | 2864 | 59 | HR | 1 & 2 | HR | 25 | 124 |
| Friberg [13] | P | Sweden | 1987–2005 | 1628 | 35,145 | 59.0i | RL & SR | 1 & 2 | RL | 22 | 203 |
| Furbergj [39] | R | Norway | 1974–1996 | 147 | 24,313 | 44.7 (6.7) | SR | 1 & 2 | RL | 0 | 130 |
| Lindemann [40] | P | Norway | 1984–2002 | 1010 | 35,751 | 49 | SR | 1 & 2 | RL | 19 | 203 |
| Ogunleye [41] | P | UK | 1993–2004 | 9577 | 19,154 | 62 | HR | 2 | RL | 13 | 17 |
| Terry [42] | R | Sweden | 1961–1992 | 142 | 10,012 | 56.2 | RL | 1 & 2 | RL | 2 | 111 |
| OR (1) | |||||||||||
| Chiou [43] | R | Taiwan | 2009–2010 | 7696 | 46,455 | 51 (18.3) | HR | 1 & 2 | HR | 70 | 306 |
| Mortality RR (2) | |||||||||||
| Campbell [44] | P | USA | 1982–2008 | 26,090 | 560,598 | – | SR | 1 & 2 | RL | 95 | 1456 |
| Coughlin [45] | P | USA | 1982–1997 | 26,186 | 562,135 | 56.22 | SR | 1 & 2 | SR | 33 | 448 |
| Mortality HR (1) | |||||||||||
| Khan [46] | P | Japan | 1988–2003 | 2363 | 61,178 | – | SR | 1&2 | RL | 1 | 21 |
| SIR (7) | |||||||||||
| Geier [47] | R | Germany | 2003–2009 | 25,380 | – | 67 | RL | 2 | RL | 61 | – |
| Hemminki [48] | P | Sweden | 1964–2007 | 125,126 | – | – | HR | 2 | RL | 374 | – |
| Ragozzino [49] | P | USA | 1945–1980 | 1135 | – | – | HR | 1 & 2 | HR | 3 | – |
| Swerdlowk [50] | P | UK | 1972–2003 | 13,212 | – | – | RL | 1 & 2 | RL | 16 | – |
| Weiderpass [27] | R | Sweden | 1977–1989 | 70,110 | – | 64.2 | HR | 1 & 2 | RL | 328 | – |
| Wideroff [51] | R | Denmark | 1977–1993 | 55,010 | – | – | HR | 1 & 2 | RL | 231 | – |
| Zendehdel [52] | P | Sweden | 1965–1999 | 29,187 | – | – | RL | 1 | RL | 12 | – |
| SMR (3) | |||||||||||
| Kessler [53] | R | USA | 1931–1960 | – | – | - | HR | 1 & 2 | HR | 44 | – |
| Swerdlowk [50] | P | UK | 1972–2003 | 13,212 | – | – | RL | 1 & 2 | RL | 5 | – |
| Verlato [56] | P | Italy | 1987–1996 | 3782 | – | 69.2 | HR | 2 | RL | 11 | – |
Abbreviations: DM: diabetes mellitus; EC: endometrial cancer; SD: standard deviation; HR: hazard ratio; RR: risk ratio; IRR: incidence rate ratio; OR: odds ratio; SIR: standardized incidence ratio; SMR: standardized mortality ratio.
R: retrospective, P: prospective.
ID: insurance database; UK: unknown; SR: self-report; HR: hospital record; HbA1c: glycated hemoglobin A1c; RL: registry linkage.
1: type 1 DM; 2: type 2 DM; UK: unknown.
Calculated based on the group means and standard deviations of age.
Median age of DM group was 63.4, interquartile range (IQR) was 58.9–67.0; median age of non-DM group was 62.2, IQR was 57.4–66.4.
The original study included both male and female subjects. 739 is the number of diabetic women enrolled.
Age range: 55–69 years.
Calculated based on the group means in the DM and control arms.
No endometrial cancer occurred in the diabetes group and the actual RR cannot be estimated.
Reported both SIR and SMR.
There were variations in the age structures of the participants and the ways in which age distributions were reported among the included studies. Among the 16 studies that reported the mean age for all participants or by exposure status, the mean age ranged from 44.7 years to 69.2 years. Standard deviations of age were missing in many of these studies. Some studies reported median ages of the participants.
Most of the studies included both T1DM and T2DM (n= 19). Eight studies focused on T2DM and one focused on T1DM, in which the authors used age younger than 30 years when hospitalized for DM as a surrogate marker of T1DM [37]. The most common methods of DM ascertainment were review of the medical charts (n = 13), patient self-report (n = 7) and DM registry (n = 5). The remaining studies which reported methods of ascertainment used insurance claims data, blood tests (glycated hemoglobin A1c) and combinations of the previously mentioned approaches. The numbers of non-diabetic controls were reported in only 17 studies, which ranged from 2864 to 895,434. In nine of the included studies the numbers of control subjects could not be identified because the incidence and mortality rate of EC among diabetic individuals were compared to those of the general population using indirect standardization.
During the follow-up, 15,912 cases of incident endometrial cancer were reported of which 2943 occurred in subjects with DM. The most common methods of ascertainment for endometrial cancer were cancer registry (n=21) and hospital pathology records (n=5). Inmost of the studies (n=23), incidence of endometrial cancer was the only primary outcome relevant to the present review and the effect measures were reported as RR (n = 7), SIR (n = 6), HR (n = 7), IRR (n = 2) or OR (n = 1). Mortality outcomes were described in five studies, which were reported as RR (n = 2), HR (n = 1) or SMR (n = 2). One study reported both SIR and SMR
Critical appraisal of the included studies
Considerable risk of bias exists in most of the included studies (see Appendix D). For example, selection bias could be introduced in studies that utilized the linkage between DM databases or hospital case records and EC registries. Because only patients hospitalized for DM were included, the study population is likely to be different from the source and target populations, which also include diabetic women managed in the ambulatory settings. Moreover, in certain other studies where individuals were invited to participate by mailed questionnaires, the response rate was as low as 16% [30], which implies significant immigrative selection bias. None of the studies detailed how they dealt with missing data or how they assessed and accounted for informative censoring, which could lead to emigrative selection bias.
The majority of the studies clearly stated in their eligibility criteria that women who had history of cancer at baseline were excluded. In addition, DM, EC and death were ascertained by hospital records, disease registries and death certificates in most studies, which are generally reliable. Nevertheless, as the included studies encompass a wide span of time, during which diagnostic criteria have evolved, there remains the possibility of misclassification [54,55]. Further, some authors have arbitrarily selected age as a surrogate marker to differentiate T1DM from T2DM, which is a fallible assumption and could have biased the results in unpredictable directions [52].
While most studies adjusted the effect measures for age, the majority of studies failed to adequately adjust for other important potential confounders that we defined a priori in the protocol (see Appendix E). The reason may be that many studies explored the associations between DM and cancers in various organs and systems and therefore adjusted for the confounding factors considered as generally important for carcinogenesis, rather than those important for EC.
Assessment of heterogeneity and meta-analysis
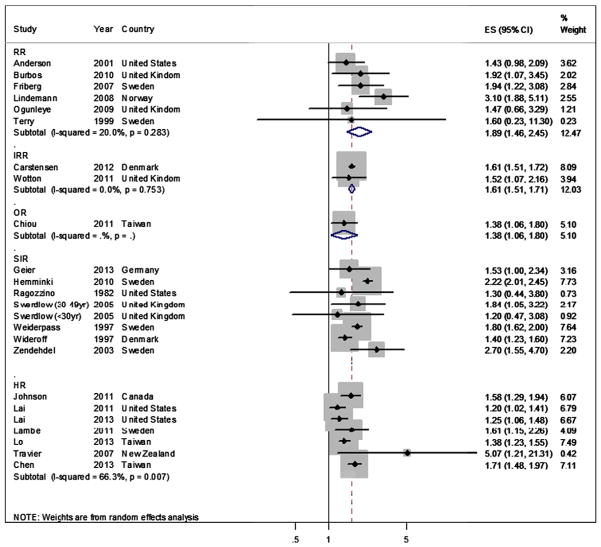
There was a considerable degree of methodological and clinical heterogeneity among the included studies, which was reflected by different study populations, study settings, lengths of follow-up and control of confounding. However, relatively low statistical heterogeneity was observed among studies reporting RR and IRR as effect measures. The I2 was 20.0% (p = 0.28) for RR and 0.0% (p = 0.75) for IRR of incident EC, respectively. Therefore, these effect measures were quantitatively synthesized. The summary RR and IRR for incident EC were 1.89 (95% CI, 1.46–2.45; p < 0.001) and 1.61 (95%CI, 1.51–1.71; p < 0.001), respectively (Fig. 2), indicating a significant increase in the risk of incident EC among diabetic women.
Fig. 2.
Meta-analysis of the adjusted incidence measures of endometrial cancer1–5. 1. Abbreviations: SIR: standardized incidence ratio; RR: relative risk; HR: hazard ratio; IRR: incidence rate ratio; OR: odds ratio; ES: effect size. 2. SIRs were derived from indirect standardization, where different reference groups were used. Therefore by definition, SIRs are not directly comparable and should not be quantitatively combined across studies. 3. HRs were not summarized due to the significant heterogeneity across studies. 4. The IRR from the study by Carstensen et al. being analyzed was imputed from the graphs, which compared cancer incidence among diabetic women using insulin to that among the non-diabetic women. 5. The percentage weight for each study was calculated as the proportion of weight it carries among all the studies regardless of the effect measures reported.
The HR estimates, on the other hand, were not synthesized due to the significant clinical, methodological and statistical heterogeneity (I2: 66.3%, p = 0.007). Moreover, statistical tests for heterogeneity were not performed for SIR or SMR because both of these two effect measures were based on different reference populations and should not be combined across studies. Nevertheless, most of the studies reporting SIR did demonstrate a positive association between DM and incident EC and all of the seven studies reporting HR showed positive associations between the two disease entities with statistical significance. In addition, the only study reporting OR also showed a 1.38 times increase (95%CI, 1.06–1.80, p = 0.018) in the risk of incident EC among the diabetic women.
Among studies that reported EC-specific mortality, only two reported RR and the I2 was 0.0% (p= 0.92), indicating low statistical heterogeneity (Fig. 3). These RR estimates were therefore synthesized and the summary estimate was 1.32 (95%CI, 1.10–1.60; p= 0.003), indicating a significant increase in the risk of EC-specific mortality among women with DM. One study reported that HR for EC-specific mortality was 1.64 (95%CI, 0.17–9.60; p = 0.58) comparing the DM group to the control group, which was not significant [46]. The other three studies [50,53,56] that reported disease-specific mortality utilized SMR as the effect measures, which could not be quantitatively synthesized. In fact, two of these studies failed to find any significant association between DM and EC-specific mortality [50,56] and the other one reported SMR (1.39) without information on confidence limit or p value [53].
Fig. 3.
Meta-analysis of the adjusted disease-specific mortality measures of endometrial cancer1–3. 1. Abbreviations: RR: relative risk; SMR: standardized mortality ratio; ES: effect size. 2. SMRs were derived from indirect standardization, where different reference groups were used. Therefore by definition, SMRs are not directly comparable and should not be quantitatively combined across studies. 3. The percentage weight for each study was calculated as the proportion of weight it carries among all the studies regardless of the effect measures reported.
The p value from the Egger’s test for small-study effects was 0.76 and the funnel plot (Fig. 4) was grossly symmetrical, both of which indicate a low probability of publication bias.
Fig. 4.
Funnel plot for all included studies1. 1. Abbreviations: RR: relative risk; HR: hazard ratio; IRR: incidence rate ratio; OR: odds ratio; SIR: standardized incidence ratio; SMR: standardized mortality ratio; Cl: confidence limit.
Discussion
In the present review, 29 cohort studies were included, most of which reported incidence of EC and only five reported data on disease-specific mortality. There was slight but significant and consistent increase in the risk of incident EC among women with DM. However, findings regarding the association between DM and EC-specific mortality were less consistent. Additionally, we did not find evidence for publication bias.
Several cohort studies and systematic reviews have demonstrated a significant increase in the risk of EC associated with pre-existing DM [12–16]. The most recent systematic review and meta-analysis on this subject was updated in June 2012 and included 21 cohort studies, in which the authors observed among diabetic women a similar increase in the risk of incident EC, but not that for EC-specific mortality [15]. In comparison to this systematic review [15], we included more studies and applied more stringent selection criteria regarding definitions of exposure and outcome, which is crucial for establishing temporality. Moreover, in the present review, both eligible prospective and retrospective cohort studies were included, in contrast to the review by Zhang et al. [15] that only included prospective cohort studies. We also searched conference abstracts to increase sensitivity of the search strategy. Notably, we did not combine SIR and SMR as these author did [15]. The reason is that indirect standardization measures are based on different reference populations and are not directly comparable. Therefore, quantitative syntheses of SIRs and SMRs are invalid and should be avoided.
In another recent systematic review for association between DM and EC, the authors included three cohort studies and thirteen case–control studies [14]. Although similar results were observed, given the significant vulnerability of case–control designs to temporal ambiguity, selection bias and recall bias, we believe that cohort study is a more valid design to answer the research question.
Although the underlying mechanisms responsible for the association between DM and EC are not fully understood, studies have shown that insulin resistance, hyperinsulinemia, hyperglycemia, inflammation and disturbances in the IGF-1 pathway may contribute to carcinogenesis among the diabetics [3], laying the foundation for biological plausibility for the causal inference. Also, as the antidiabetic agent metformin has been shown to reduce incidence of various types of cancers compared to insulin or sulfonylureas [16,57], it remains to be studied as to whether similar effects could be observed in EC and whether glucose control versus other pleiotropic mechanisms may have played a role.
There are several limitations in the present review. First, there remains the possibility that there were relevant conference proceedings not yet uncovered in our search. We may have failed to include some relevant studies by restricting the search to studies published in English. Nevertheless, the extensive hand-searching helped us to identify relevant studies and improved the coverage of our search. Moreover, we hand-searched conference abstracts of the last three years assuming that studies reported in earlier conferences would have been reported as journal articles, which would be covered by the extensive database searching. Second, as discussed previously, limitations in designs and conduct of the included studies, such as selection bias, misclassification bias and residual confounding, compromise validity. However, we have closely examined each included study and attempted to identify their major limitations to aid interpretation of the results. Third, heterogeneity in study design, conduct and analysis, which is pervasive among observational studies on DM and EC, challenges quantitative synthesis of the effect measures. However, despite considerable clinical and methodological heterogeneity, we have not observed significant statistical heterogeneity in some of the risk estimates and therefore have synthesized the results accordingly. In contrast, in one of the previous meta-analyses, the authors integrated the effect measures despite the apparent clinical, methodological and statistical heterogeneity, the last of which was indicated by an I2 as high as 94.1% [15]. Furthermore, due to the significant variations in the ways with which the authors reported the original results, we were not able to conduct sensitivity or subgroup analysis. Finally, it is noteworthy that in many relevant studies a large variety of cancer outcomes were concurrently investigated and the significant results found with such a “shot-gun” approach should be interpreted with caution.
The present review provides a systematic evaluation and synthesis of the up-to-date evidence and further demonstrates the causal association between DM and incident EC. Such findings can inform screening and management of EC in diabetic women and further motivate prevention and control of diabetes. However, despite the well-recognized advantages of systematic reviews and meta-analyses, quality of the original studies remains the most fundamental determinant of the quality of the synthesized evidence. To further disentangle the association between DM and EC, investigators should better conceptualize the causal framework and adequately consider confounding, selection bias and information bias in future studies. In addition, regarding the long and insidious process preceding cancer onset, case cohort studies nested in established cohorts and clinical trials may be a more efficient design to answer the research question, which was listed in our eligibility criteria but was not adopted by any relevant studies that we were able to identify.
In conclusion, the present study suggests a significantly increased risk of incident EC among women affected by DM. Evidence on the associations between DM and EC-specific mortality is less consistent. In light of the limitations and heterogeneity in the original studies, understanding of the relationship between the two disease entities will continue to benefit from high-quality epidemiologic studies. Future studies should pay particular attention to selection of participants, control of important confounders, ascertainment of DM and EC, and proper handling of sample attrition and missing data. Factors that can potentially modify the associations between DM and incidence or mortality of EC, such as DM duration, anti-diabetic drugs and glucose control, should also be taken into consideration.
Supplementary Material
HIGHLIGHTS.
We performed a systematic review and meta-analysis of 29 cohort studies.
Diabetes significantly increases the risk of incident endometrial cancer (EC).
Data on the association between diabetes and EC-specific mortality are inconsistent.
Acknowledgments
Sources of funding
This study itself did not receive any funding. Part of Dr. Tompkins’ salary was supported by National Institute on Drug Abuse (NIDA; DA029609) during the conduct of this systematic review.
We gratefully acknowledge Dr. Kay Dickersin, Dr. Tianjing Li and Dr. Ian Saldanha for their constructive advice towards this project. We also thank Mr. Rob Wright for his assistance in formatting the electronic search strategies.
Appendix. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2014.07.095.
Footnotes
Conflict of interest statement
None of the investigators have any conflict of interest.
References
- 1. [Last accessed on 2014, Feb 19th]; http://www.idf.org/diabetesatlas.
- 2.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2011 Nov 8;8(4):228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 3.Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 2013 Jan;104(1):9–14. doi: 10.1111/cas.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011 Sep;47(13):1928–37. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011 Nov;26(11):863–76. doi: 10.1007/s10654-011-9617-y. [DOI] [PubMed] [Google Scholar]
- 6.Huang W, Ren H, Ben Q, Cai Q, Zhu W, Li Z. Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control. 2012 Feb;23(2):263–72. doi: 10.1007/s10552-011-9874-9. [DOI] [PubMed] [Google Scholar]
- 7.Ge Z, Ben Q, Qian J, Wang Y, Li Y. Diabetes mellitus and risk of gastric cancer: a systematic review and meta-analysis of observational studies. Eur J Gastroenterol Hepatol. 2011 Nov;23(12):1127–35. doi: 10.1097/MEG.0b013e32834b8d73. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012 Apr 1;130(7):1639–48. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 9.Ren HB, Yu T, Liu C, Li YQ. Diabetes mellitus and increased risk of biliary tract cancer: systematic review and meta-analysis. Cancer Causes Control. 2011 Jun;22(6):837–47. doi: 10.1007/s10552-011-9754-3. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011 May;54(5):1013–8. doi: 10.1007/s00125-011-2051-6. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007 Aug 15;121(4):856–62. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 12.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008 Dec 17;300(23):2754–64. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friberg E, Mantzoros CS, Wolk A. Diabetes and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2007 Feb;16(2):276–80. doi: 10.1158/1055-9965.EPI-06-0751. [DOI] [PubMed] [Google Scholar]
- 14.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007 Jul;50(7):1365–74. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZH, Su PY, Hao JH, Sun YH. The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: a meta-analysis of prospective cohort studies. Int J Gynecol Cancer. 2013 Feb;23(2):294–303. doi: 10.1097/IGC.0b013e31827b8430. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes—a systematic review and meta-analysis. Diabetes Obes Metab. 2014 Aug;16(8):707–10. doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 17.Zhou XH, Qiao Q, Zethelius B, Pyorala K, Soderberg S, Pajak A, et al. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010 Sep;53(9):1867–76. doi: 10.1007/s00125-010-1796-7. [DOI] [PubMed] [Google Scholar]
- 18.Stattin P, Bjor O, Ferrari P, Lukanova A, Lenner P, Lindahl B, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007 Mar;30(3):561–7. doi: 10.2337/dc06-0922. [DOI] [PubMed] [Google Scholar]
- 19.Verlato G, Drane JW, Aldrich TE. Statistical methods for surveillance of diabetes events. Diabetes Nutr Metab. 2003 Jun;16(3):198–200. [PubMed] [Google Scholar]
- 20.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Google Scholar]
- 21.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013 Jul 10;31(20):2607–18. doi: 10.1200/JCO.2012.48.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]: the Cochrane collaboration. 2011. [Google Scholar]
- 24.Loannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. Can Med Assoc J. 2007;176(8):1091–6. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adami HO, McLaughlin J, Ekbom A, Berne C, Silverman D, Hacker D, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991 Sep;2(5):307–14. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 27.Weiderpass E, Gridley G, Persson I, Nyren O, Ekbom A, Adami HO. Risk of endometrial and breast cancer in patients with diabetes mellitus. Int J Cancer. 1997 May 2;71(3):360–3. doi: 10.1002/(sici)1097-0215(19970502)71:3<360::aid-ijc9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Chen HF, Liu MD, Chen P, Chen LH, Chang YH, Wen PC, et al. Risks of breast and endometrial cancer in women with diabetes: a population-based cohort study. PLoS One. 2013 Jun 27;8(6):e67420. doi: 10.1371/journal.pone.0067420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia. 2011 Sep;54(9):2263–71. doi: 10.1007/s00125-011-2242-1. [DOI] [PubMed] [Google Scholar]
- 30.Lai GY, Park Y, Hartge P, Hollenbeck AR, Freedman ND. The association between self-reported diabetes and cancer incidence in the NIH-AARP Diet and Health Study. J Clin Endocrinol Metab. 2013 Mar;98(3):E497–502. doi: 10.1210/jc.2012-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai G, Park Y, Hartge P, Hollenbeck A, Schatzkin A, Freedman N. The association between diabetes and cancer incidence and mortality in the NIH-AARP study. Cancer Res. 2011;71(8) [Google Scholar]
- 32.Lambe M, Wigertz A, Garmo H, Walldius G, Jungner I, Hammar N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes Control. 2011 Aug;22(8):1163–71. doi: 10.1007/s10552-011-9794-8. [DOI] [PubMed] [Google Scholar]
- 33.Lo SF, Chang SN, Muo CH, Chen SY, Liao FY, Dee SW, et al. Modest increase in risk of specific types of cancer types in type 2 diabetes mellitus patients. Int J Cancer. 2013 Jan 1;132(1):182–8. doi: 10.1002/ijc.27597. [DOI] [PubMed] [Google Scholar]
- 34.Travier N, Jeffreys M, Brewer N, Wright CS, Cunningham CW, Hornell J, et al. Association between glycosylated hemoglobin and cancer risk: a New Zealand linkage study. Ann Oncol. 2007 Aug;18(8):1414–9. doi: 10.1093/annonc/mdm135. [DOI] [PubMed] [Google Scholar]
- 35.Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia. 2012 Apr;55(4):948–58. doi: 10.1007/s00125-011-2381-4. [DOI] [PubMed] [Google Scholar]
- 36.Wotton CJ, Yeates DG, Goldacre MJ. Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia. 2011 Mar;54(3):527–34. doi: 10.1007/s00125-010-1987-2. [DOI] [PubMed] [Google Scholar]
- 37.Anderson KE, Anderson E, Mink PJ, Hong CP, Kushi LH, Sellers TA, et al. Diabetes and endometrial cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2001 Jun;10(6):611–6. [PubMed] [Google Scholar]
- 38.Burbos N, Musonda P, Giarenis I, Shiner AM, Giamougiannis P, Morris EP, et al. Predicting the risk of endometrial cancer in postmenopausal women presenting with vaginal bleeding: the Norwich DEFAB risk assessment tool. Br J Cancer. 2010 Apr 13;102(8):1201–6. doi: 10.1038/sj.bjc.6605620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer. 2003 May 10;104(6):669–76. doi: 10.1002/ijc.10974. [DOI] [PubMed] [Google Scholar]
- 40.Lindemann K, Vatten LJ, Ellstrom-Engh M, Eskild A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer. 2008 May 6;98(9):1582–5. doi: 10.1038/sj.bjc.6604313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogunleye AA, Ogston SA, Morris AD, Evans JM. A cohort study of the risk of cancer associated with type 2 diabetes. Br J Cancer. 2009;101(7):1199–201. doi: 10.1038/sj.bjc.6605240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terry P, Baron JA, Weiderpass E, Yuen J, Lichtenstein P, Nyren O. Lifestyle and endometrial cancer risk: a cohort study from the Swedish Twin Registry. Int J Cancer. 1999 Jul 2;82(1):38–42. doi: 10.1002/(sici)1097-0215(19990702)82:1<38::aid-ijc8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 43.Chiou WK, Huang BY, Chou WY, Weng HF, Lin JD. Incidences of cancers in diabetic and non-diabetic hospitalized adult patients in Taiwan. Asian Pac J Cancer Prev. 2011;12(6):1577–81. [PubMed] [Google Scholar]
- 44.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012 Sep;35(9):1835–44. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004 Jun 15;159(12):1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 46.Khan M, Mori M, Sakauchi F, Aklimunnessa K, Kubo T, Fujino Y, et al. Risk of endometrial cancer mortality by ever-use of sex hormones and other factors in Japan. Asian Pac J Cancer Prev. 2006 Apr-Jun;7(2):260–6. [PubMed] [Google Scholar]
- 47.Geier AS, Wellmann J, Wellmann I, Kajuter H, Heidinger O, Hempel G, et al. Cancer detection rates following enrolment in a disease management programme for type 2 diabetes. Diabetologia. 2013 Sep;56(9):1944–8. doi: 10.1007/s00125-013-2947-4. [DOI] [PubMed] [Google Scholar]
- 48.Hemminki K, Li X, Sundquist J, Sundquist K. Risk of cancer following hospitalization for type 2 diabetes. Oncologist. 2010;15(6):548–55. doi: 10.1634/theoncologist.2009-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragozzino M, Melton LJ, 3rd, Chu CP, Palumbo PJ. Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J Chronic Dis. 1982;35(1):13–9. doi: 10.1016/0021-9681(82)90025-x. [DOI] [PubMed] [Google Scholar]
- 50.Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, et al. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. 2005 Jun 6;92(11):2070–5. doi: 10.1038/sj.bjc.6602611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997 Sep 17;89(18):1360–5. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 52.Zendehdel K, Nyren O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003 Dec 3;95(23):1797–800. doi: 10.1093/jnci/djg105. [DOI] [PubMed] [Google Scholar]
- 53.Kessler II. Cancer mortality among diabetics. J Natl Cancer Inst. 1970 Mar;44(3):673–86. [PubMed] [Google Scholar]
- 54.Harris MI, Hadden WC, Knowler WC, Bennett PH. International criteria for the diagnosis of diabetes and impaired glucose tolerance. Diabetes Care. 1985 Nov-Dec;8(6):562–7. doi: 10.2337/diacare.8.6.562. [DOI] [PubMed] [Google Scholar]
- 55.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verlato G, Zoppini G, Bonora E, Muggeo M. Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care. 2003 Apr;26(4):1047–51. doi: 10.2337/diacare.26.4.1047. [DOI] [PubMed] [Google Scholar]
- 57.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009 Sep;32(9):1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.