Abstract
Periodic outbreaks of spruce budworm (SBW) affect large areas of ecologically and economically important conifer forests in North America, causing tree mortality and reduced forest productivity. Host resistance against SBW has been linked to growth phenology and the chemical composition of foliage, but the underlying molecular mechanisms and population variation are largely unknown. Using a genomics approach, we discovered a β-glucosidase gene, Pgβglu-1, whose expression levels and function underpin natural resistance to SBW in mature white spruce (Picea glauca) trees. In phenotypically resistant trees, Pgβglu-1 transcripts were up to 1000 times more abundant than in non-resistant trees and were highly enriched in foliage. The encoded PgβGLU-1 enzyme catalysed the cleavage of acetophenone sugar conjugates to release the aglycons piceol and pungenol. These aglycons were previously shown to be active against SBW. Levels of Pgβglu-1 transcripts and biologically active acetophenone aglycons were substantially different between resistant and non-resistant trees over time, were positively correlated with each other and were highly variable in a natural white spruce population. These results suggest that expression of Pgβglu-1 and accumulation of acetophenone aglycons is a constitutive defence mechanism in white spruce. The progeny of resistant trees had higher Pgβglu-1 gene expression than non-resistant progeny, indicating that the trait is heritable. With reported increases in the intensity of SBW outbreaks, influenced by climate, variation of Pgβglu-1 transcript expression, PgβGLU-1 enzyme activity and acetophenone accumulation may serve as resistance markers to better predict impacts of SBW in both managed and wild spruce populations.
Keywords: Picea glauca, insect resistance, conifer forest health, acetophenone biosynthesis, glucosyl hydrolase, transcriptome profiling
Introduction
Outbreaks of insect herbivores can cause widespread mortality of forest trees and drastically alter landscapes. Examples of forest pests with large outbreak dynamics and severe impacts on their environments include the spruce budworm (SBW) (Gray and MacKinnon, 2006), the mountain pine beetle (Kurz et al., 2008; Raffa et al., 2013) and the emerald ash borer (Poland and McCullough, 2006). The SBW [Choristoneura fumiferana (Clem)] is a lepidopteron native to eastern North America that defoliates conifers, primarily mature spruce (Picea) and fir (Abies) trees (Blais, 1983) (Figure1a). The SBW outbreak of 1950–1993 covered an area of 850 000 km2 in Canada, killed 45–58% of tree hosts in highly affected areas and decreased wood yields by 300–6800 m3 km−2 (Gray and MacKinnon, 2006). Efforts to predict future occurrences and model the potential impacts of SBW outbreaks indicate that they will be exacerbated by changing environmental conditions (Gray and MacKinnon, 2006), similar to the recent spread of the mountain pine beetle epidemic (Kurz et al., 2008; Raffa et al., 2013). An understanding of mechanisms of resistance to the SBW, including its molecular underpinnings, may enhance our ability to predict and potentially mitigate the impacts of SBW and evaluate the ability of host trees to acclimate and adapt to changing conditions.
Figure 1.
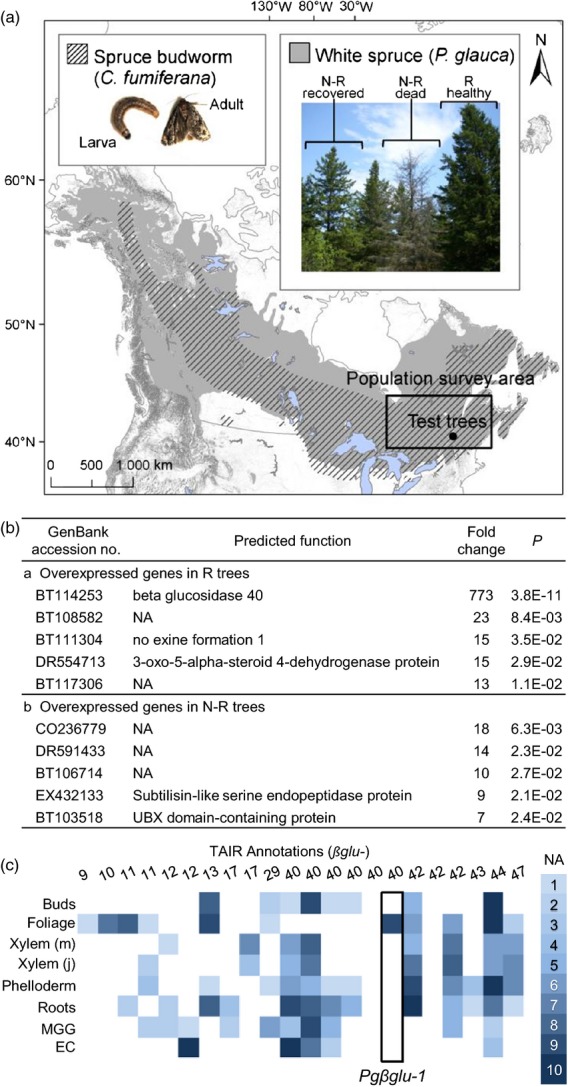
Experimental system and expression of the Pgβglu-1 gene.(a) Distribution of white spruce (Picea glauca) and spruce budworm (Choristoneura fumiferana; SBW). [Distribution map accessed at https://cfs.nrcan.gc.ca/projects/107 (30 March 2014) and reproduced with permission from the Natural Resources Canada/Canadian Forest Service.] The black circle and square show the test trees site and the population survey area, respectively. In the SBW box: larval stage 6 and adult moth. In the white spruce box: resistant (R) and recovering non-resistant (N-R) phenotypes shown in 2010 about 3 years after a local spruce budworm outbreak.(b) Genes with largest differences in transcript abundance in the foliage of R (n = 7) and N-R trees (n = 7), identified by using an oligonucleotide microarray.(c) The relative expression of glycosyl hydrolase family 1 transcripts was compared across eight tissues: vegetative buds (buds), foliage, xylem mature (m), xylem juvenile (j), phelloderm, roots, megagametophyte (MGG) and embryonic culture (EC). Putative glycosyl hydrolase transcripts are annotated with the closest Arabidopsis match found in the TAIR database (http://www.arabidopsis.org/). Expression in foliage was measured in 4-year-old trees not previously exposed to SBW.
Host resistance against SBW has been linked to growth phenology and the chemical composition of foliage (Clancy, 2002; Daoust et al., 2010; Delvas et al., 2011), but the underlying mechanisms and population patterns of variation are largely unknown. Constitutive and inducible defence mechanisms against insects have been identified in herbaceous plants like Nicotiana attenuata and Arabidopsis thaliana (Kessler and Baldwin, 2002; Todesco et al., 2010; Züst et al., 2012) and in forest trees such as spruce (Hall et al., 2011) and poplar (Irmisch et al., 2013). In forest trees, an understanding of the molecular mechanisms underlying variations in insect resistance has begun to develop (Robert et al., 2010; Hall et al., 2011) and is expected to be key to determining their ability to survive and adapt, when considering their high levels of variability across populations, wide-ranging distributions and changing environmental conditions.
As forward and reverse genetic techniques can be difficult to apply in non-model species and are poorly developed for mature forest trees, we used an alternative approach based on comparative transcriptome profiling of resistant (R) and non-resistant (N-R) trees in a forest setting to discover genes involved in SBW resistance in white spruce (Picea glauca (Voss.) Moench.). Mature white spruce trees with contrasting resistance levels were previously identified during a localized SBW outbreak (1998–2007) (Figure1a) (Daoust et al., 2010). Average annual defoliation intensities served to identify trees as R (0–20% defoliation) or N-R (30–70% defoliation). At the end of the outbreak, R trees had remained healthy whereas N-R trees were dead or had recovered from defoliation but had sparse foliage (Figure1a). The foliage of R trees accumulated high levels of the acetophenone metabolites piceol and pungenol as well as the corresponding glucosides, picein and pungenin (Figure2). In contrast, the foliage of N-R trees contained predominantly the acetophenone glucosides and very low levels of the aglycons (Delvas et al., 2011). Compared with controls, laboratory-reared SBW fed with piceol and pungenol at concentrations found in the needles of R trees showed survival rates reduced by up to 50%, slower development and lower pupal mass (Delvas et al., 2011).
Figure 2.
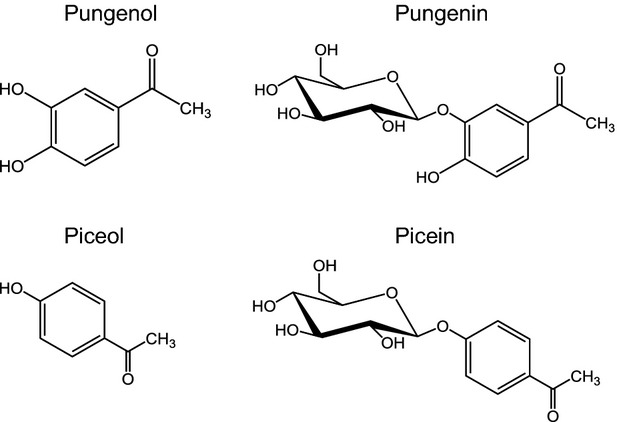
The structures of biologically active acetophenone aglycons, pungenol and piceol, and their respective glucosides, pungenin and picein.
Comparative transcriptome screening of mature R and N-R white spruce trees, reported here, revealed a glucosyl hydrolase gene, Pgβglu-1, highly expressed in R trees. We identified and characterized the corresponding enzyme function and variation in gene expression, which explained differences in the acetophenone aglycon levels in R and N-R trees. We monitored Pgβglu-1 transcript profiles over time and between years, its inheritance and its geographic origins within a white spruce population. In summary, we report a β-glucosidase gene of plant acetophenone metabolism, whose expression and function explain differences in acetophenone levels associated with SBW resistance in white spruce.
Results
Transcriptome profiling identifies Pgβglu-1 as highly expressed in SBW-resistant trees
We screened the expression of nearly 24 000 different white spruce genes in the foliage of R and N-R trees. Transcripts of 236 genes were more abundant in R trees, while transcripts of 250 genes were more abundant in N-R trees (P < 0.05) (Table S1 in Supporting Information). Transcript levels for most of the genes varied less than 10-fold between R and N-R trees. In contrast, transcripts corresponding to a gene identified here as Pgβglu-1 (GenBank BT114253) were 770 times more abundant in current-year foliage of R trees than in N-R trees (Figure1b). Transcripts of only one other gene of unknown function varied more than 20-fold (Figure1b). Validation by quantitative reverse transcriptase polymerase chain reaction assay (RT-qPCR) showed Pgβglu-1 transcript abundance about 1000-fold higher in R compared with N-R trees, with the same samples as used with the microarray (Mann–Whitney–Wilcoxon, P = 0.0015).
Full-length cDNA and genomic sequences of Pgβglu-1
We cloned and sequenced the full-length (FL) Pgβglu-1 complementary DNA (cDNA) (GenBank KJ780719) and the corresponding genomic DNA. The translated Pgβglu-1 FL cDNA sequence encodes a PgβGLU-1 protein 506 amino acids long (Figure S2) which contains a glycosyl hydrolase family 1 domain characteristic for enzymes that hydrolyse phenolic glucosides, disaccharides or other glycosylated substrates (Hill and Reilly, 2008) and shares 52% of amino acids with the A. thaliana enzyme β-glucosidase 40.
Pgβglu-1 is preferentially expressed in foliage
The white spruce gene catalogue, which represents 27 720 unique sequences (Rigault et al., 2011), has 242 sequences containing a glycosyl hydrolase domain (33 different PFAM domains); 22 of these contained a glycosyl hydrolase family 1 domain most similar to plant β-glucosidases. Of these sequences, Pgβglu-1 had the highest expression among those that appeared to be selectively expressed in foliage (Figure1c; data from the PiceaGeneExpress database; Raherison et al., 2012).
Assessment of Pgβglu-1 sequence variation in R and N-R trees
We identified intact Pgβglu-1 genes in both R and N-R trees. Sequence variations were screened over 3598 bp including exons (13) and introns (12) as well as upstream and downstream regions. Comparing genomic Pgβglu-1 sequences from seven R and seven N-R trees, we identified a total of 19 polymorphic sites in the translated protein sequences. However, none of the variations were specific to either R or N-R trees (Table S2), and none of the variations affected the conserved functional sites (Figure S2). Thus, these variations would not easily explain the observed chemical differences in R and N-R trees. None of the trees were homozygous nonsense Pgβglu-1 mutants.
Differential Pgβglu-1 expression correlates with levels of biologically active acetophenones
We monitored levels of Pgβglu-1 transcripts, the acetophenones piceol and pungenol and the acetophenone glucoside picein over two growth seasons, 3 and 6 years after SBW outbreak. Levels of Pgβglu-1 transcripts, piceol and pungenol were higher in R than N-R trees in June or August in both years (Figure3a–c). Pgβglu-1 expression was positively correlated with piceol (Pearson's correlation, r = 0.66, P < 0.001) and pungenol (Pearson's correlation, r = 0.68, P < 0.001) and negatively correlated with picein (Pearson's correlation, r = −0.39, P = 0.02). Piceol and pungenol levels were positively correlated (Pearson's correlation, r = 0.84, P < 0.001). These correlations are as expected for putative substrate and products of the predicted β-glucosidase enzyme activity of the Pgβglu-1 gene product.
Figure 3.
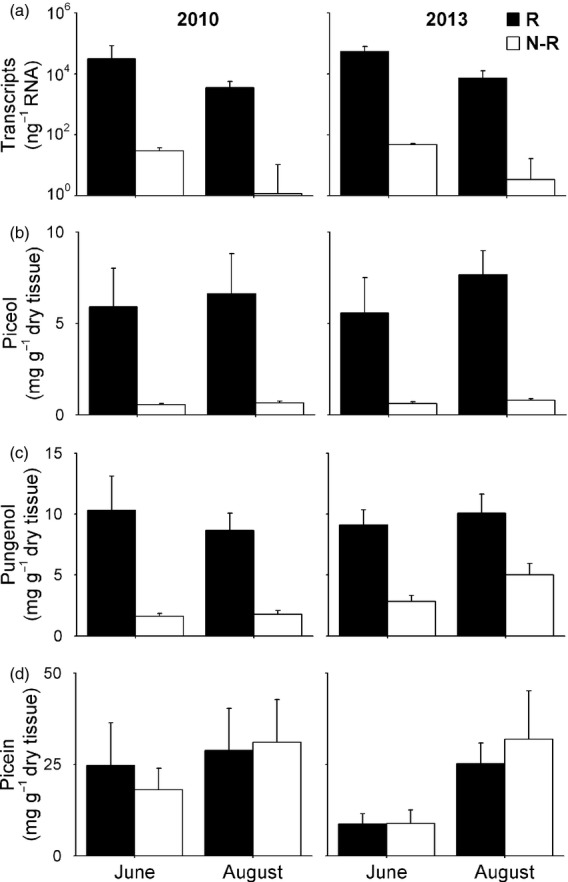
Differential Pgβglu-1 expression and acetophenones levels were maintained over time. Foliar phenotypes of test trees during 2010 (n = 8) and 2013 (n = 10) of resistant (R) and non-resistant trees (N-R). We report results of mixed model analysis with year, month, resistance phenotype as fixed effects and with their interactions.(a) Pgβglu-1 transcript levels were higher in R than N-R trees (F1,26 = 92.17, P < 0.001) and in June than August (F1,26 = 10.58, P = 0.003). (B–D) Acetophenone concentrations from R and N-R trees. Piceol and pungenol levels are higher in R than in N-R trees (piceol F1,28 = 37.77, P < 0.001, pungenol F1,28 = 50.42, P < 0.001). Picein levels did not differ between resistance phenotypes (F1,28 = 0.01, ns) but varied between months (F1,28 = 5.04, P = 0.03). All panels show mean ± SEM.
Predicted properties and structural features of PgβGLU-1
The predicted PgβGLU-1 protein has a theoretical isoelectric point (pI) of 5.57, an N-terminal signal peptide (score of 0.915 out of 1) and a cleavage site between amino acids 25 and 26 (Figure S2) (SignalP 4.1 server; Petersen et al., 2011), and appears to be targeted to the secretory pathway (targetP v1.1 software; secretory pathway score 0.966 out of a maximum of 1; scores for mitochondrion, chloroplast and others were below 0.124; secretory pathway reliability class = 1 out of 5, where 1 is highest; Emanuelsson et al., 2000). wolfpsort and multiloc software (Höglund et al., 2006; Horton et al., 2007) indicated that PgβGLU-1 is a vacuolar protein (vacuolar score of 0.92 out of 1; scores for extracellular matrix and endoplasmic reticulum were below 0.05). These predictions suggest that PgβGLU-1 is targeted to the vacuole and may be released to its lumen after cleavage of a hydrophobic signal peptide.
We used protein modelling for initial assessment of possible interactions of PgβGLU-1 with the glucosylated acetophenones picein and pungenin. PgβGLU-1 was predicted to have a (β/α)8-barrel fold similar to other GH1 enzymes (Figure4a) with two catalytic glutamate residues (Sansenya et al., 2011). Amino acids of the modelled active site (Figure4b) interacting with the sugar moiety are highly conserved (Marana, 2006), while amino acids interacting with the aglycone component are less conserved. The modelled Michaelis complex with picein suggested H-bonding with the catalytic glutamate residues (Figure4b). The methyl and oxygen functionalities of the phenolic group of picein are positioned in the hydrophobic and positively charged regions, respectively, of the predicted GH1 aglycone subsite (Figure4c).
Figure 4.
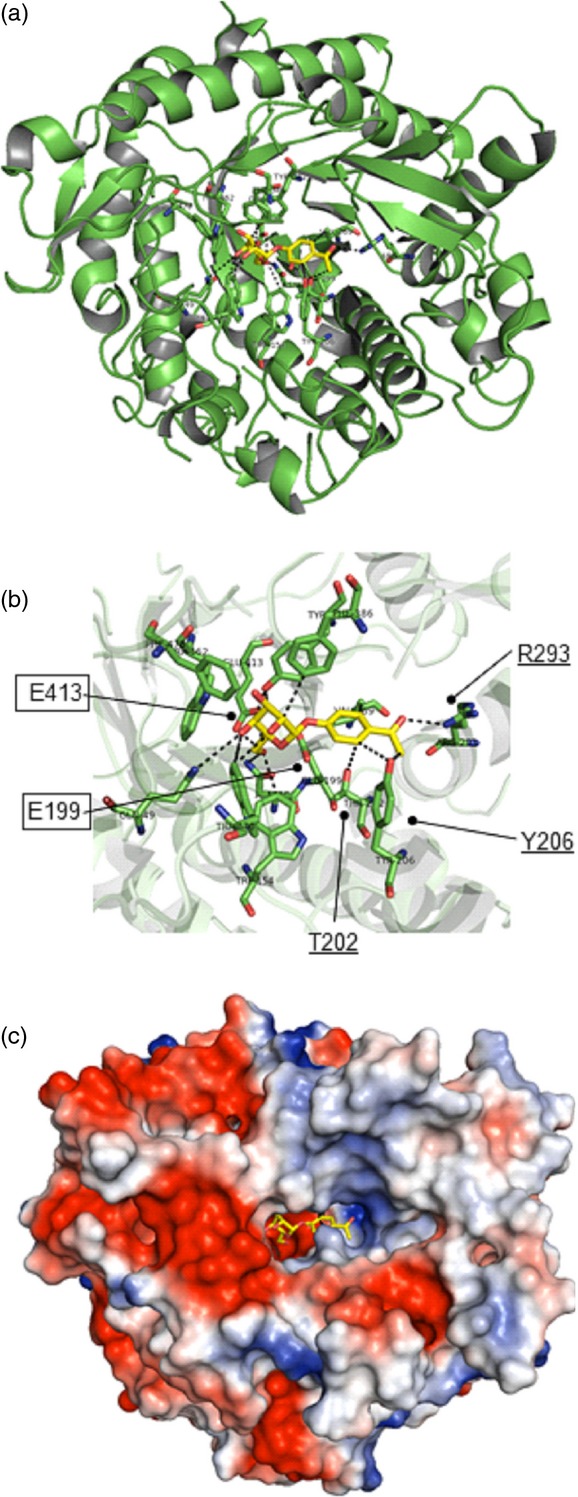
Structural model of the Pgβglu-1 gene product.(a) Cartoon representation of the overall structure of the PgβGLU-1 protein.(b) Predicted PgβGLU-1 Michaelis complex with picein. Catalytic site glutamic acid residues (boxed, E199, E413): nucleophile E413 Oe1 was hydrogen bonded (dashed lines) with O5 (2.92 Å) and the distance between Oe2 of the catalytic acid/base residue E199 and O1 of picein is 3.35 Å. Three amino acids (underlined, T202, Y206, R293) predicted to form hydrogen bonds with the phenolic moiety of picein.(c) Electrostatic potential surface representation, negatively and positively charged areas in red and blue, respectively.
PgβGLU-1 protein catalyses the formation of biologically active acetophenones
To substantiate putative functions with biochemical evidence, recombinant PgβGLU-1 protein was produced in, and purified from, Nicotiana benthamiana leaves, which can serve as a reliable expression system for plant proteins that are difficult to produce in microbial hosts. PgβGLU-1 enzyme was active with both picein and pungenin, forming the respective aglycons (Figure5a). The enzyme kinetic parameters of PgβGLU-1 were determined in assays with 0.01–2.0 mm picein. Km, kcat and kcat/Km were 1.28 ± 0.53 mm, 0.41 sec−1 and 0.32 mm−1 sec−1, respectively. The kinetic parameters are within the range of previously characterized A. thaliana β-glucosidases BGLU45 and BGLU46 (Escamilla-Treviño et al., 2006). PgβGLU-1 showed high level of specificity for picein compared with the following glucosides with similar structures: 4-nitrophenyl-glucoside, vanillin 4-O-β-glucoside, salicin, arbutin and benzyl-β-glucoside (Figure5b). Together, these results established the glucosides picein and pungenin as substrates and the aglycons piceol and pungenol as products of PgβGLU-1 enzyme activity.
Figure 5.
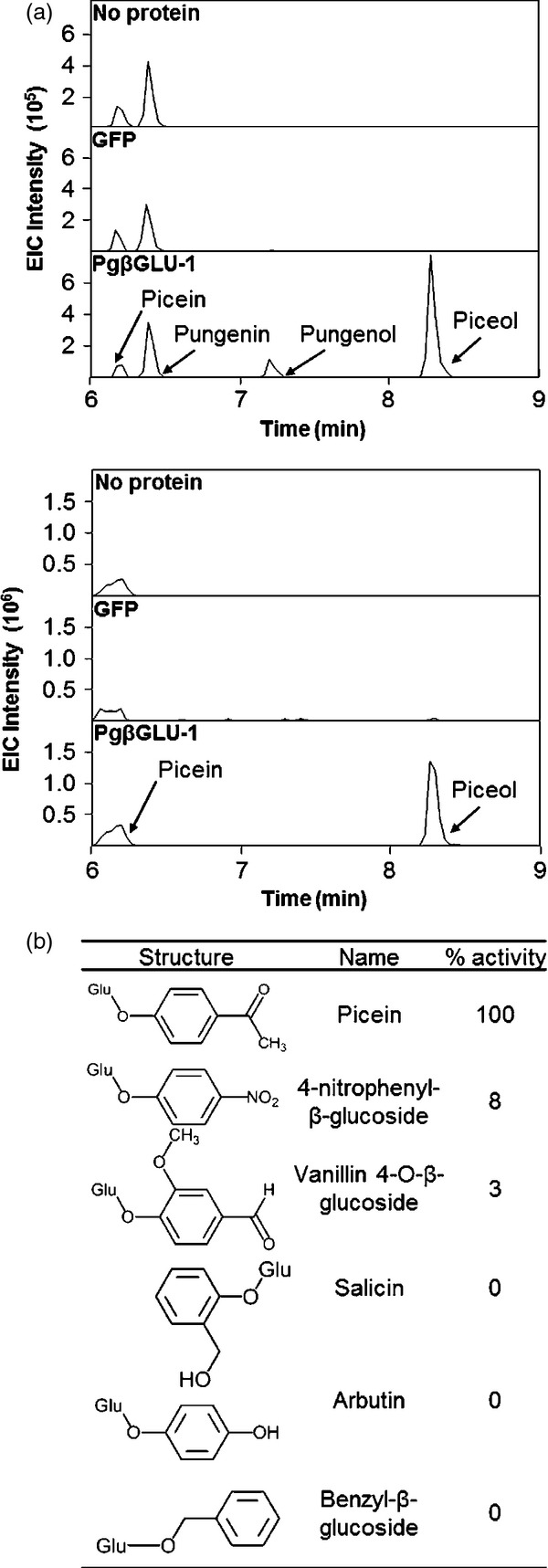
Enzyme activity of recombinant PgβGLU-1 protein.(a) Liquid chromatography mass spectrometry (LCMS) profiles for PgβGLU-1 enzyme assays. Recombinant PgβGLU-1 was assayed by incubating purified protein with an extract from white spruce needles (top) or 0.1 mm picein (bottom). Note that pungenin was not commercially available for testing. The LCMS profiles are shown as extracted ion chromatograms in positive mode for the parent ion of pungenol (peak 3) and pungenin (peak 2) (152 + 1; dashed line) or piceol (peak 4) and picein (peak 1) (136 + 1; continuous line). Controls were performed with no protein or recombinant GFP protein.(b) The relative activity of PgβGLU-1 on other phenylpropanoid glucosides was compared to picein. Little or no activity was observed with compounds other than picein.
Variation of resistance traits in the white spruce population and inheritance of expression level
Variability of Pgβglu-1 expression, piceol, pungenol and picein was analysed with an independent set of genetically unrelated trees from 23 locations covering a large geographic area in the eastern range of white spruce (De Lafontaine et al., 2010; Figure1a, Table S3) which were grown in a common garden with no observed defoliation by SBW. The levels of Pgβglu-1 transcripts and acetophenones varied widely (Figure6a–d). The highest observed levels surpassed those of test R trees by 1.3-fold and two-fold for Pgβglu-1 expression and both of the aglycons, respectively, and the lowest levels of the same traits were similar to the N-R trees. Several trees had intermediate levels of transcripts or acetophenones, consistent with continuous distributions of the traits in the population. Abundance of Pgβglu-1 transcripts was positively correlated with piceol (Pearson's correlation, r = 0.43, P = 0.011) and pungenol levels (Pearson's correlation, r = 0.45, P = 0.006) as observed in test R and N-R trees but no correlation was observed between levels of Pgβglu-1 transcripts and picein (Pearson's correlation, r = −0.23, not significant). Piceol and pungenol levels were also positively correlated (Pearson's correlation, r = 0.51, P = 0.002).
Figure 6.
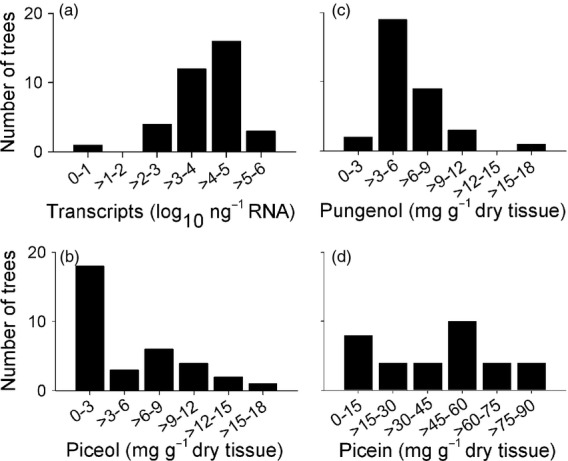
Resistance trait variations in white spruce population at the end of the growth season. Distribution of Pgβglu-1 expression (a), piceol (b), pungenol (c) and picein (d) into abundance classes determined as six equal fractions of the maximal level for each trait. The analysis used 15-year-old trees from a common garden not previously exposed to spruce budworm.
Inheritance of Pgβglu-1 gene expression levels was evaluated in 1-year-old progeny of R and N-R test trees grown in controlled conditions with no exposure to SBW. Transcript accumulation was on average 20-fold higher in R progeny than N-R progeny (Figure7). Expression levels varied more widely in the R tree progeny, as expected for plants grown from wind-pollinated seeds where the paternal parent is unknown. Expression levels in young trees were also much lower than observed in mature parent trees, which was not surprising as SBW defoliation occurs mostly in mature trees.
Figure 7.
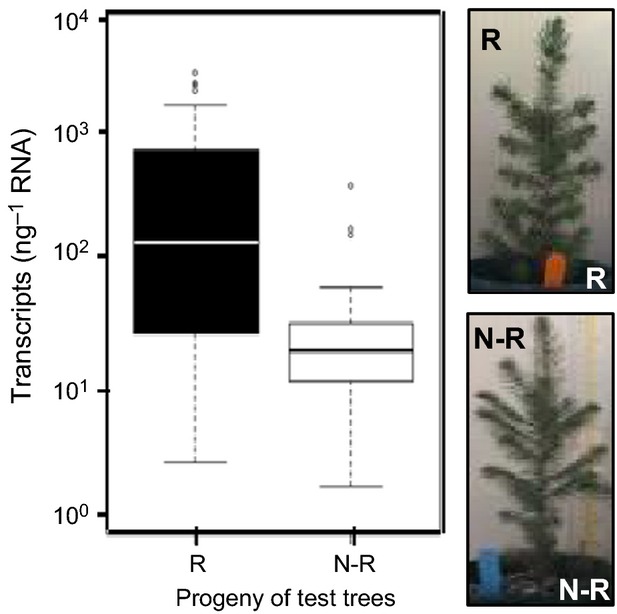
Inheritance of Pgβglu-1 expression in young progeny of resistant (R) and non-resistant (N-R) parent trees.Differential expression in the foliage of 1-year-old trees in wind-pollinated families derived from R (n = 80 trees, four families) and N-R (n = 80 trees, four families) mother trees (Mann–Whitney–Wilcoxon test, P = 4.13 × 10−5). Trees were grown in the greenhouse and not exposed to spruce budworm.
Discussion
Using a genomics approach including transcriptome and gene expression analyses and biochemical functional characterization, we discovered a white spruce Pgβglu-1 gene and identified its function as a control point in accumulation of acetophenone in SBW resistance. Prior to their implication in SBW resistance (Delvas et al., 2011), biological activities of acetophenones in tree defence were not known, although previous work linked the ratio of piceol to picein to general defences and abiotic stress in spruce (Hoque, 1985; Lokke, 1990) and piceol to pathogen resistance in some herbaceous plant species (Curir et al., 1996). One of the acetophenone glucosides, pungenin, was tested on SBW and found to be a modest feeding deterrent for sixth-instar larvae, but no effect was found on development or mortality (Strunz et al., 1986). In contrast, the aglycons retarded development and increased mortality (Delvas et al., 2011). The upstream biosynthetic route for acetophenones was previously proposed (Negrel and Javelle, 2010) (Figure8, step 1), but the enzymes or genes of this pathway had not been described. Variation in the level of accumulation of biologically active acetophenones in SBW R and N-R white spruce trees appears to be determined by glucosyl hydrolysis of acetophenone glucosides, which is controlled by Pgβglu-1 transcript levels and the encoded PgβGLU-1 enzyme activity (Figure8, step 3). The PgβGLU-1 enzyme catalyses the release of both piceol and pungenol, and their levels of accumulation were correlated both to each other and to the levels of Pgβglu-1 transcript in the natural population, indicating that they are likely to be products of the same enzyme activity. Availability of the glucoside precursor (Figure8, step 2) does not appear to be rate-limiting for a release of aglycons, since in N-R trees levels of piceol are low despite high levels of picein (Figure3). Full assessment of the stoichiometry of acetophenone aglycon and glucoside pools requires future discovery and characterization of the corresponding glucosyltransferase(s).
Figure 8.
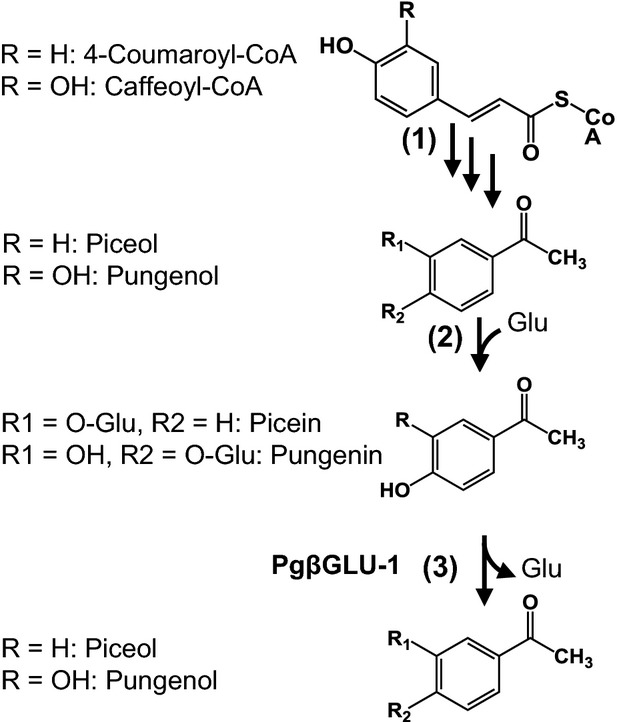
Schematic of a proposed biosynthetic pathway for the hydroxylacetophenones piceol and pungenol and their respective glucosides picein and pugenin.The proposed biosynthesis of piceol and pungenol involves, respectively, coumaroyl-CoA or caffeolyl CoA as precursors (1) (Negrel and Javelle, 2010). This is followed by glucosylation (2) to produce the glucosides picein and pungenin which are found in resistant and non-resistant trees. Deglucosylation by PgβGLU-1 releases the aglycons in resistant trees. The schematic does not account for different compartments, membranes or different cell types potentially involved in the formation, transport and accumulation of the glucosides and release of aglycons.
The tissue and cellular localization of piceol, pungenol and their glucosides, as well as the localization of PgβGLU-1 and relevant glucosyltransferase enzymes are not known. Many plant defence molecules are glycosylated and stored in the vacuole, while the β-glucosidases that may activate and release them are localized in a different part of the cell or tissue (Morant et al., 2008). Unlike, for example, cyanogenic glucosides or glucosinolates, which come into contact with their bio-activating β-glucosidases upon tissue disruption, acetophenone aglycons were found in spruce tissues that had not been exposed to mechanical damage prior to sampling. This suggests that the acetophenone glucosides come into contact with β-glucosidase enzymes without tissue disruption. Whether the glucosides and PgβGLU-1 are both contained in the same compartment, potentially the vacuole, or transported to a specialized cell type or some other location is unknown and warrants further investigation. High levels of acetophenone aglycon may be toxic to the tissues producing them (Hoque, 1985). It is therefore possible that acetophenones are first produced as glucosides allowing them to be transported to the vacuole and are then released by PgβGLU-1. This type of scheme where the bulk of acetophenones are initially glucosylated and then released may allow for tighter control over the concentration and containment of potentially harmful aglycons in the cell.
Here we showed that genetically heritable variation of expression of a single Pgβglu-1 gene with preferential expression in foliage underpins the resistance of white spruce to defoliation by SBW. The accumulation of acetophenones in newly formed foliage of mature trees was found several years after previous exposure to the SBW, and was also observed in trees of broad geographic origins for which there was no obvious prior exposure to SBW. Therefore, accumulation of the acetophenone aglycons as a defence appears to be constitutive in white spruce in contrast to other glucosyl hydrolase-mediated defence systems, which invoke glycoside hydrolysis upon tissue damage for the release of active defence compounds from precursors such as cyanogenic glucosides or glucosinolates (Morant et al., 2008). Insect defence mechanisms characterized in several other conifer species are both constitutive and inducible. Resistance of lodgepole pine against the mountain pine beetle involves quantitative multigenic control (Yanchuk et al., 2008), and may be based on a suite of chemical and physical defences (Franceschi et al., 2005; Bohlmann, 2012). In Sitka spruce, resistance to the white pine weevil has been associated with variations of stone cells and resin ducts (King et al., 2011) and constitutive and induced terpenoid levels and their underlying terpenoid synthases (Hall et al., 2011; Roach et al., 2014). The present study showed that levels of both Pgβglu-1 transcripts and acetophenone aglycons active against the SBW are elevated in early summer when the most destructive SBW larval stages are active (Miller, 1977). The constitutive production of acetophenone aglycons provides an immediate defence against ravenous SBW larvae. We did not investigate whether Pgβglu-1 transcript or protein expression is inducible and if induction could afford phenotypic plasticity of SBW resistance.
The differences of expression of Pgβglu-1 in R and N-R trees suggest the presence of variation of this gene in cis regulatory sequences, or a variation of trans-acting factors. Variation in the coding sequences of Pgβglu-1 could not explain the observed phenotypic differences. Phenotypic variation within and among species result from coding sequence and regulatory mutations but some types of variations are more likely to result from regulatory mutations because of the inherent properties of transcription (Wray, 2007). Transcription is modular and allows fine-tuning. Regulatory mutations tend to be dominant or additive. Hence, expression variation is usually less pleiotropic and more directly visible to selection than coding variation (Wray, 2007; Galego Romero et al., 2012). Recent studies have shown that variations in gene expression contribute to evolutionary changes in systems ranging from crop plants (Chen et al., 2013; Koenig et al., 2013) to animals and human diseases (Romero et al., 2012). Through transcriptional variation and high outbreeding, tree populations may accumulate diverse alleles that enable adaptation to changing conditions over long periods of time.
Outbreaks of SBW have increased in frequency and scale in the last two centuries (Blais, 1983) and a potentially large outbreak is currently emerging in eastern Canada (NRCAN, http://www.nrcan.gc.ca/forests/insects-diseases/13383). Combating SBW with insecticides may be a short-term solution to reduce damage but is costly to deploy and has met with environmental concerns. While hundreds of millions of white spruce trees are planted in North America every year for forest renewal and to boost wood production, an outbreak of SBW could cancel out potential yield improvements within its range. The molecular underpinning of resistance to SBW with differential expression of a Pgβglu-1 gene and its encoded substrate-specific β-glucosidase activity described here could enable breeding for resistance to SBW. Incorporation of knowledge about resistance mechanisms and their molecular underpinning may also improve existing models of SBW spread and risk assessment.
Experimental procedures
Plant material and sampling
Test trees
Picea glauca trees were identified as phenotypically R or N-R to SBW defoliation in a plantation established in 1963 and located at Saint-Cyrille-de-Wendover, Québec, Canada (45°93′N, 72°52′W) (Daoust et al., 2010). Plantation trees were grown from open-pollinated seeds from local white spruce populations. Resistance phenotypes were based on levels of SBW defoliation (R, 0–20%; N-R, 30–70%) during a local outbreak (1998–2007) and field rearing of SBW in sleeve cages on the trees (Daoust et al., 2010). A total of seven R and eight N-R trees were selected from the study of Delvas et al. (2011). All trees were 47 years old at the start of the study in 2010. Current-year foliage was sampled in 2010 on 17 June and 6 August. For each tree, three different foliage samples were taken from different parts of the live crown and used for separate analyses. One sample was from the top third of the live crown and two samples from different branches in the central third of the crown. In 2013, five R and five N-R trees were sampled on 18 June and 13 August and current-year foliage was sampled from the central third of the crown.
Progeny of test trees
Half-sib progeny of four R and four N-R trees were obtained by growing plants from open-pollinated seeds that were collected in September 2011 (120–200 seeds per tree), cleaned and stored at −20°C. Seeds were germinated and seedlings were grown in high-density polyethylene 110 ml pots, a potting mix of peat, perlite and vermiculite (60, 20, 20%, respectively) and fertilized weekly with 20/20/20. Plants were grown in a plastic greenhouse from June 2012 to April 2013, which represents an extended growth period, and then placed in cooled growth rooms for a simulated dormancy period of 13 weeks. Plants were transferred to 1.7 L pots in July 2013 and placed in an open-end plastic house for growth. Two weeks after the transfer, foliage samples comprising newly formed foliage and 1-year-old needles were taken from the eight open-pollinated families (20 trees per family).
Population trees
White spruce trees originating from 23 different locations were sampled in a common garden established in 1999 in Valcartier, Québec, Canada (46°56′N, 71°29′W) (Table S3). A total of 360 genotypes were available in the common garden and had been selected in genetic progeny tests of white spruce established with seeds from locations throughout the Province of Québec and are part of a tree breeding programme. In the population survey, we selected one tree randomly for each of the locations. In 2013, current-year leaves were collected from the central third of the crown of 79 trees on 3 July (early during growth season) and 1 October (late during growth season).
All foliage samples were flash frozen in liquid N2 immediately after removal from the trees and stored at −80°C. Foliage was ground to a fine powder using a MixerMill 300 (Retsch, http://www.retsch.com/) and steel grinding balls cooled in nitrogen. Powdered tissue was stored at −80°C until extraction for metabolite and RNA analyses.
Transcriptome analyses
Total RNA was extracted as described (Chang et al., 1993) with modifications (Pavy et al., 2008) and stored at −80°C. The total RNA concentration was determined using a NanoDrop 1000 (Thermo Scientific, http://www.thermoscientific.com/) and assessed for quality with an Agilent 2100 Bioanalyzer and Agilent RNA 6000 Nano Kit LabChips (Agilent Technologies Inc., http://www.agilent.com/). Transcriptome profiling was carried out with RNA extracted from seven R and seven N-R trees sampled in 2012 (17 June). All three samples per tree (total n = 42) were analysed with a custom microarray comprising oligonucleotide probes for 23 853 unique P. glauca gene sequences (Raherison et al., 2012). Hybridizations were performed using HS 400® Pro Hybridization Stations (TECAN, http://www.tecan.com/). Before use in hybridization, microarray slides were washed with 0.5× saline-sodium citrate (SSC), 0.1% sodium dodecyl sulphate (SDS) at 37°C and with 2× SSC, 0.5% SDS at 50°C for 20 sec each. They were pre-hybridized for 1 h with medium agitation at 65°C in a pre-hybridization buffer (5× SSC, 0.1% SDS, 0.2 mg ml−1 BSA and 0.1 mg ml−1 herring sperm DNA) to prevent non-specific binding. Slides were washed twice with 2× SSC, 0.5% SDS. The RNA probes were prepared for hybridization by using Amino Allyl Message Amp® II aRNA Amplification Kit following the manufacturer's instructions (Applied Biosystems/Ambion, http://www.lifetechnologies.com/) and labelled with Alexa Fluor® 555 (Molecular Probes Inc., http://www.lifetechnologies.com/). The number of dye molecules incorporated per 1000 nucleotides was calculated by measuring absorbance on a NanoDrop 1000 (Thermo Scientific) (http://www.nanodrop.com/). Only samples with more than 30 dye molecules per 1000 nucleotides proceeded to the next steps. Labelled RNA samples were fragmented using RNA Fragmentation Reagents following the manufacturer's instructions (Applied Biosystems/Ambion), denatured for 2 min at 65°C, cooled on ice for 1 min and centrifuged at 13 000 g for 30 sec. A 120 μl volume of hybridization buffer (50% formamide, 5× SSC, 0.1% SDS and 0.1 mg ml−1 herring sperm DNA) pre-heated to 46°C was added to each sample for hybridization and samples were kept at 46°C in a heating block until they were added to the slides. Hybridization solutions were injected onto each slide and hybridized at 45°C for 16 h with medium agitation. Slides were washed twice with 2× SSC, 0.5% SDS at 45°C, three times with 0.5× SSC, 0.1% SDS, respectively at 45, 37 and 23°C, twice with 0.1× SSC at 23°C and once with Nanopure water at 23°C. Slides were then dried using pressurized nitrogen at 39 p.s.i. for 2 min 30 sec. Slides were scanned the same day in a PowerScanner® (TECAN) at 5 μm resolution. Laser power was set to 50% for all of the slides but the photomultiplier (PMT) was adjusted for each slide using an autogain function. Hybridization signals were converted to raw intensities with ArrayPro® Analyser version 6.3 software (MediaCybernetics, http://www.mediacy.com/). Median intensity and local background were obtained for each spot on the array. Bad spots resulting from dust, high local background or saturation were flagged. Net intensity was calculated by subtracting the trimmed background from median intensity and was used for further analysis.
Data analysis was carried out using the flexarray 1.6 software package (http://genomequebec.mcgill.ca/FlexArray). A-quantile normalization was used to normalize intensity distributions between arrays. Statistically significant variation was determined by univariate analysis of variance, corrected for multiple testing (Benjamini and Hochberg, 1995). The list of differentially expressed genes is given in Table S1. Gene identification and annotations were as previously described (Rigault et al., 2011). Tissue expression of white spruce sequences similar to known plant β-glucosidase genes was extracted from the PiceaGeneExpress database (Raherison et al., 2012).
Full length cloning of the Pgβglu-1 cDNA
A β-glucosidase gene sequence was identified by microarray transcriptome profiling (Pgβglu-1); however, the longest available cDNA clone (GenBank BT114253) contained only a partial protein-coding sequence based on similarity searches to public databases. Analysis of RNA-Seq data (Rigault et al., 2011) produced a putative full-length (FL) RNA transcript of 2118 nucleotides (nt) with a 5′ untranslated region (UTR) of 177 nt and a 3′ UTR of 423 nt. Primers were designed to match the ends of the putative FL RNA sequence (Table S4); a cDNA fragment was amplified by PCR from one of the R trees and cloned using a TOPO TA Cloning® Kit for sequencing (Invitrogen, http://www.invitrogen.com/). Briefly, Platinum® Taq DNA Polymerase High Fidelity (Invitrogen) was used following the manufacturer's instructions. The amplified product was purified using the PCR Purification Kit (Invitrogen). Then, 1.25 units of Platinum® Taq DNA Polymerase (Invitrogen), 1× Platinum® Taq PCR buffer minus Mg, 1.5 mm MgCl2 and 1 mm of dATP were added, and the reaction was incubated for 10 min at 72°C for post-amplification addition of 3′ A- overhangs. The sample was placed on ice and the TOPO® cloning reaction was started immediately following the manufacturer's instructions (Invitrogen) with 15 min incubation at 24°C. Transformation was done with OneShot® Chemically Competent Escherichia coli (Invitrogen). The DNA from positive clones was purified using QIAprep® (Qiagen, http://www.qiagen.com/) and a colony-PCR was performed to verify the length of the insert before sequence analysis. Alignment with previously obtained sequences used the BioEdit Sequence Alignment Editor and sequence similarity searches were carried out with BLAST. The resulting cDNA was 1761 nt and the predicted protein coding sequence was 1518 nt or 506 amino acids, a 5′ UTR of 58 nt and a 3′ UTR of 183 nt (GenBank KJ780719). It contained a coding sequence that was identical to previous sequences for the gene.
Reverse transcriptase-qPCR analysis
Reverse transcriptase-qPCR with gene-specific primers (Table S4) was used to validate microarray results in the mature R (n = 5) and N-R (n = 8) test trees. Primers were designed using Primer3Plus software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi), self-complementarity of the newly designed primers was verified using Oligo Calc: Oligonucleotide Properties Calculator software (http://www.basic.northwestern.edu/biotools/oligocalc.html) and specificity was verified against the P. glauca gene catalogue (Rigault et al., 2011). Complementary DNA synthesis used 500 ng of total RNA and the Superscript® First-Strand cDNA synthesis system for RT-PCR (Invitrogen). Resulting cDNAs were diluted 1:4 in RNase-free water before qPCR quantification. The PCR mixtures were composed with a QuantiFast® SYBR® Green PCR kit (Qiagen) as follows: 1× master mix, 300 nm of 5′ and 3′ primers and 5 μl of cDNA in a final volume of 15 μl. Amplifications were carried out in a LightCycler® 480 (Roche, http://www.roche.com/) as described in Boyle et al. (2009). The LRE method (Rutledge and Stewart, 2008) adapted for Excel (Boyle et al., 2009) was used to calculate the number of transcript molecules, which was normalized to a ratio calculated by the geometric mean of three reference genes: elongation factor 1a (EF1-α) (BT102965), cell division cycle 2 (CDC2) (BT106071) and ribosomal protein L3A (BT115036) as described elsewhere (Beaulieu et al., 2013).
Amplification specificity was assed based on melting curve analyses and by amplicon sequencing. For RT-qPCR in N-R trees, preliminary analyses showed that amplicons were produced which contained intronic sequences. Intron-containing amplicons were specific to N-R trees and the Pgβglu-1 gene, and were greatly decreased by DNase I treatment of the RNA samples. To prevent potential confounding effects associated with residual genomic DNA, all of the primers used to assess transcript levels were positioned such as to span the junction of two exons.
To verify that RT-qPCR assay was robust when analysing genetically diverse individuals, three different primer pairs were designed and tested in test trees and in our survey of natural population trees. The results from all three primers pairs were highly correlated for the population trees, indicating high repeatability of results (Figure S1).
Identification of the genomic sequence of Pgβglu-1
Genomic DNA was extracted using DNeasy Plant Mini Kit (Qiagen). The DNA concentrations were determined using a NanoDrop 1000 (Thermo Scientific) and DNA integrity was assessed with agarose gel electrophoresis. Primers for the Pgβglu-1 gene (BT114253) were designed as described for RT-PCR. Using genomic DNA sampled in 2010 for all the test trees, PCR amplification was conducted with Platinum Taq DNA polymerase High Fidelity (LifeTechnologies, https://www.lifetechnologies.com/). The PCR programme was: 5 min at 94°C followed by 35 cycles of 15 sec at 94°C and 1 min at 62°C and a final elongation step of 1 min at 68°C. Amplicons were purified with ExoSAP-IT® for PCR Product Cleanup (Affymetrix, http://www.affymetrix.com/) and Sanger sequenced. Sequences were aligned and analysed with BioEdit software (http://www.mbio.ncsu.edu/bioedit/bioedit.html). The sequence assembly was performed using the chromaspro 1.5 software (http://www.technelysium.com.au/ChromasPro.html). A unique consensus sequence was obtained by retaining the most frequent nucleotide at each site. Sites within sequences were identified as polymorphic with at least one variant out of 14 trees.
Protein modelling and substrate docking
A PgβGLU-1 protein model was built based on the crystal structure of Os4BGLU12 (Sansenya et al., 2011) using the modelling software nest from the Jackal package (http://wiki.c2b2.columbia.edu/honiglab_public/index.php/Software:Jackal). Model quality was assessed by analysis of a Ramachandran plot through PROCHECK (Laskowski et al., 1993). The one- or two-dimensional structures of picein, pungenin, piceol and pungeol were obtained from the PDB, PubChem and Zinc databases. Their three-dimensional structure was modelled with Babel software (http://openbabel.org/wiki/Main_Page). Substrate docking of picein and pungenin in the three-dimensional PgβGLU-1 protein model was performed with the software autodock vina (Trott and Olson, 2010) and results interpreted based on reports for substrate specificity in family 1 glycoside hydrolases and plant β-glucosidases (Verdoucq et al., 2004; Marana, 2006) by using a grid with dimensions of 30 Å × 30 Å × 30 Å centred on the catalytic acid/base (E199) and nucleophile (E413) residues. Images were generated using pymol (http://www.pymol.org/). The theoretical pI of PgβGLU-1 was determined (http://web.expasy.org/compute_pi/). Targeting of the PgβGLU-1 protein was assessed by predicting signal peptides with the software signalP (Peterson et al., 2001), whether it is secreted with targetP (Emanuelsson et al., 2000) and its localization with woLF PSORT (Horton et al., 2007) and multiloc (Höglund et al., 2006).
Protein expression and purification
Pgβglu-1 without the signal peptide was cloned into the GoldenGate ready-expression vector pEAQ-GG, and fused to secreted SlCYS8-tag with a Factor Xa cleavable linker (Sainsbury et al., 2009, 2013). This construct, as well as pEAQ-GG-GFP, was transformed into Agrobacterium tumefaciens stain AGL1 for N. benthamiana expression. For tissue infiltration, bacteria grown at 28°C under agitation in kanamycin-containing Luria–Bertani (LB) culture medium were harvested by centrifugation at 2400 g for 10 min, and resuspended in filtration medium [10 mm 2-(N-morpholine)-ethanesulphonic acid (MES) buffer, pH 5.8, 10 mm MgCl2] obtaining an OD600 of 0.5. The bacterial suspensions were blended with an equal volume of A. tumefaciens cells harbouring protein p19, a gene-silencing suppressor from the tomato bushy stunt virus (Voinnet et al., 2003). The bacterial mixtures were put in 1-ml needle-free syringes and gently forced into the abaxial side of fully expanded N. benthamiana leaves. After 5 days, leaves were harvested, flash frozen and stored at −80°C. Leaves were weighed and ground in liquid nitrogen. Five millilitres of extraction buffer (50 mm MES-KOH, pH 6; 50 mm NaCl) per gram fresh weight was added to the tissue and tissue was incubated at 4°C with shaking for 1 h. Supernatant was collected by centrifuging for 15 min at 3000 g and freezing overnight at −80°C to precipitate Rubisco protein. The protein extract was defrosted in a water bath and centrifuged for 15 min at 3000 g. Imidazole (20 mm) and DTT (1 mm) were added to supernatant. Prepared Ni-NTA resin (Qiagen) was added and extracts were incubated at 4°C with shaking for 1 h. Protein was purified using a gravity column. The column was washed with buffer (50 mm MES-KOH, pH 6; 150 mm NaCl; 20 mm imidazole; 1 mm DTT) and eluted by incubating the resin for 10 min with elution buffer (50 mm MES-KOH, pH 6; 150 mm NaCl; 150 mm imidazole; 1 mm DTT) before collecting protein. Protein was desalted using a PD-25 desalting columns (GE Lifesciences, http://www.gelifesciences.com/) and desalting buffer (50 mm MES-KOH, pH 6; 10% glycerol). Protein was quantified using Bradford reagent (Bio-Rad, http://www.bio-rad.com/). The secretion SlCYS8 tag was cleaved by incubating the protein overnight at 16°C with Factor Xa (1 μl per 250 μl of protein).
Βeta-glucosidase enzyme assays
Enzyme assays were performed using reaction buffer (20 mm MES-KOH, pH 6), 0.01–2.0 mm picein (Toronto Research Chemicals Inc., http://www.trc-canada.com/) or foliar methanolic extract, 10 μl purified protein and 100 μm benzoic acid as an internal standard in a total reaction volume of 200 μl. Assays were incubated for 1 h at 37°C then stopped with ethyl acetate and vortexed well. After centrifugation for 10 min at 1000 g, 300 μl of the top layer was place in a new vial and dried under nitrogen gas. Assays were resuspended in 100 μl of 100% methanol and analysed using LC-MS. Relative activity assays were performed as above except that 0.025 mm of the various substrates was used. A LC-MS analysis was performed using a LC-MSD-Trap-XCT_plus with a 15-cm SB-C18 column (Agilent Technologies Inc.). Solvent A was water with 0.2% (v/v) formic acid; solvent B was 100% (v/v) acetonitrile with 0.2% (v/v) formic acid. The following gradient was used: increase to 5% solvent B from 0 to 0.5 min; increase to 22% solvent B from 0.5 to 5.0 min; increase to 35% solvent B from 5.0 to 10.0 min; increase to 50% solvent B from 10.0 to 13.0 min; increase to 95% solvent B from 13.0 to 16.0 min; holding 95% solvent B from 16.0 to 17.0 min; decrease to 5% solvent B from 17.0 to 17.1 min. Column flow rate was 0.8 ml min−1. Picein, piceol, pungenin and pungenol were identified using the parent mass for the aglycons piceol and pungenol, 137(+) and 153(+) respectively. Also, authentic standards of picein, piceol and pungenol were used to verify the identities (Toronto Research Chemicals Inc.; Sigma-Aldrich; TCI America, Portland, http://www.tcichemicals.com/). Pungenin is not commercially available.
Extraction and HPLC analysis of phenolic compounds
Phenolic compounds were extracted using 50–100 mg of fine needle powder with 70% aqueous methanol HPLC grade as the solvent. Benzoic acid at 1 mg ml−1 was used as an internal standard. Aqueous methanol (600 μl) was added to the needle powder and incubated at 4°C on an agitation plate for 48 h. Aqueous methanol was removed (after centrifugation at 13 000 g for 10 min) and preserved (−80°C) after 6, 24 and 48 h of incubation. After 6 and 24 h of incubation, a fresh 600 μl of aqueous methanol was added to each sample to continue the extraction. Extracts obtained after 6, 24 and 48 h were pooled as a single extract. The Varian Prostar HPLC used for quantification was equipped with a UV detector 325, autosampler 420 and a solvent delivery module 240. Acetophenones were separated through a pre-column Polaris MetaGuard 4.6 mm and a column Polaris 250 mm × 4.6 mm C18-A (Agilent Technologies Inc.) both heated at 62°C. The solvent and solvent gradient were identical to those used in the enzyme assay. The column flow rate was 1.5 ml min−1. Ten microlitres of extract diluted 1:2 in solvent A was injected. Quantification was done using calibration curves for picein, piceol and pungenol.
Acknowledgments
The authors thank P. Huron, G. Tétrault, D. St-Pierre, M. Lamara, P. Lenz and L. Madilao for technical assistance; C. Goulet and F. Sainsbury for pEAQ-GG constructs and the Agrobacterium strain; B. Schneider, C. Paetz, M. Fischer and S. Withers for their assistance in chemical structure confirmation; and D. Baulcombe for the pEAQ-p19 construct. The work was supported by the IFOR Research Consortium; the Natural Sciences and Engineering Research Council of Canada (NSERC; Strategic Project Grant to EB, JB, JJM); and funds from Genome Canada, Genome BC and Genome Québec for the SMarTForests Project (to JB, JJM). JB is a UBC Distinguished University Scholar.
Conflict of interest
The authors confirm that they have no conflict of interest to be declared in accordance with the journal policy.
Accession numbers
Sequence data for Pgβglu-1 are available in GenBank (GenBank KJ780719) and transcriptome profiling data are available in the Gene Expression Omnibus (GEO, acc. GSE57301).
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Validation of RT-quantitative PCR assay.
Predicted amino acid sequence of PgβGLU-1 protein.
Differentially expressed genes between resistant (R) and non-resistant (N-R) trees.
Variations in Pgβglu-1 amino acid sequence of resistant (R) and non-resistant (N-R) trees.
White spruce provenances sampled for the population survey.
Primer sequences used for quantitative PCR analysis, molecular cloning, and sequencing assays.
References
- Beaulieu J, Giguère I, Deslauriers M, Boyle B, MacKay J. Differential gene expression patterns in white spruce newly formed tissue on board the International Space Station. Adv. Space Res. 2013;52:760–772. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B. 1995;57:289–300. [Google Scholar]
- Blais J. Trends in the frequency, extent and severity of spruce budworm outbreaks in eastern Canada. Can. J. For. Res. 1983;13:539–547. [Google Scholar]
- Bohlmann J. Pine terpenoid defences in the mountain pine beetle epidemic and in other conifer pest interactions: specialized enemies are eating holes into a diverse, dynamic and durable defence system. Tree Physiol. 2012;32:943–945. doi: 10.1093/treephys/tps065. [DOI] [PubMed] [Google Scholar]
- Boyle B, Dallaire N, MacKay J. Evaluation of the impact of single nucleotide polymorphisms and primer mismatches on quantitative PCR. BMC Biotechnol. 2009;9:75. doi: 10.1186/1472-6750-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993;11:113–116. [Google Scholar]
- Chen T, Lv Y, Zhao T, Li N, Yang Y, Yu W, He X, Liu T, Zhang B. Comparative transcriptome profiling of a resistant vs. susceptible tomato (Solanum lycopersicum) cultivar in response to infection by tomato yellow leaf curl virus. PLoS ONE. 2013;18:e80816. doi: 10.1371/journal.pone.0080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy K. Mechanisms of resistance in trees to defoliators. In: Wagner M, Clancy K, Lieutier F, Paine T, editors. Mechanisms and Deployment of Resistance in Trees to Insects. Dordrecht, The Netherlands: Kluwer; 2002. pp. 77–101. eds). [Google Scholar]
- Curir P, Marchesini A, Danieli B, Mariani F. 3-Hydroxyacetophenone in carnations is a phytoanticipin active against Fusarium oxysporum f. sp. dianthi. Phytochemistry. 1996;41:447–450. [Google Scholar]
- Daoust SP, Mader BJ, Bauce E, Despland E, Dussutour A, Albert PJ. Influence of epicuticular-wax composition on the feeding pattern of a phytophagous insect: implications for host resistance. Canad. Entomol. 2010;142:261–270. [Google Scholar]
- Delvas N, Bauce É, Labbé C, Ollevier T, Bélanger R. Phenolic compounds that confer resistance to spruce budworm. Entomol. Exp. Appl. 2011;141:35–44. [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- Escamilla-Treviño LL, Chen W, Card ML, Shih M-C, Cheng C-L, Poulton JE. Arabidopsis thaliana β-Glucosidases BGLU45 and BGLU46 hydrolyse monolignol glucosides. Phytochemistry. 2006;67:1651–1660. doi: 10.1016/j.phytochem.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Krokene P, Christiansen E, Krekling T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005;167:353–375. doi: 10.1111/j.1469-8137.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- Gray D, MacKinnon W. Outbreak patterns of the spruce budworm and their impacts in Canada. For. Chron. 2006;82:550–561. [Google Scholar]
- Hall DE, Robert JA, Keeling CI, Domanski D, Qesada AL, Jancsik S, Kuzyk M, Hamberger BR, Borchers CH, Bohlmann J. An integrated genomic, proteomic and biochemical analysis of (+)-3-carene biosynthesis in Sitka spruce (Picea sitchensis) genotypes that are resistant or susceptible to white pine weevil. Plant J. 2011;65:936–948. doi: 10.1111/j.1365-313X.2010.04478.x. [DOI] [PubMed] [Google Scholar]
- Hill AD, Reilly PJ. Computational analysis of glycoside hydrolase family 1 specificities. Biopolymers. 2008;89:1021–1031. doi: 10.1002/bip.21052. [DOI] [PubMed] [Google Scholar]
- Höglund A, Dönnes P, Blum T, Adolph H-W, Kohlbacher O. MultiLoc: prediction of protein subcellular localization using N-terminal targeting sequences, sequence motifs and amino acid composition. Bioinformatics. 2006;22:1158–1165. doi: 10.1093/bioinformatics/btl002. [DOI] [PubMed] [Google Scholar]
- Hoque E. Norway spruce dieback: occurrence, isolation and biological activity of p-hydroxy acetophenone and p-hydroxy acetophenone-O-glucoside and their possible roles during stress phenomena. Eur. J. For. Path. 1985;15:129–145. [Google Scholar]
- Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmisch S, McCormick AC, Boeckler GA, et al. Two herbivore-induced cytochrome P450 enzymes CYP79D6 and CYP79D7 catalyze the formation of volatile aldoximes involved in poplar defense. Plant Cell. 2013;25:4737–4754. doi: 10.1105/tpc.113.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Plant responses to insect herbivory: the emerging molecular analysis. Annu. Rev. Plant Biol. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- King JN, Alfaro RI, Lopez MG, Van Akker L. Resistance of Sitka spruce (Picea sitchensis (Bong.) Carr.) to white pine weevil (Pissodes strobi Peck): characterizing the bark defence mechanisms of resistant populations. Forestry. 2011;84:83–91. [Google Scholar]
- Koenig D, Jiménez-Gómez JM, Kimura S, et al. Comparative transcriptomics reveals patterns of selection in domesticated and wild tomato. Proc. Natl Acad. Sci. USA. 2013;110:E2655–E2662. doi: 10.1073/pnas.1309606110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L. Mountain pine beetle and forest carbon feedback to climate change. Nature. 2008;452:987–990. doi: 10.1038/nature06777. [DOI] [PubMed] [Google Scholar]
- De Lafontaine G, Turgeon J, Payette S. Phylogeography of white spruce (Picea glauca) in eastern North America reveals contrasting ecological trajectories. J. Biogeogr. 2010;37:741–751. [Google Scholar]
- Laskowski R, Macarthur M, Moss D, Thornton J. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993;26:283–291. [Google Scholar]
- Lokke H. Picein and piceol concentrations in Norway spruce. Ecotoxicol. Environ. Saf. 1990;19:301–309. doi: 10.1016/0147-6513(90)90032-z. [DOI] [PubMed] [Google Scholar]
- Marana SR. Molecular basis of substrate specificity in family 1 glycoside hydrolases. IUBMB Life. 2006;58:63–73. doi: 10.1080/15216540600617156. [DOI] [PubMed] [Google Scholar]
- Miller C. The feeding impact of spruce budworm on balsam fir. Can. J. For. Res. 1977;7:76–84. [Google Scholar]
- Morant AV, Jorgensen K, Jorgensen C, Paquette SM, Sanchez-Perez R, Moller BL, Bak S. Β-Glucosidases as detonators of plant chemical defense. Phytochemistry. 2008;69:1795–1813. doi: 10.1016/j.phytochem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Negrel J, Javelle F. The biosynthesis of acetovanillone in tobacco cell-suspension cultures. Phytochemistry. 2010;71:751–759. doi: 10.1016/j.phytochem.2010.01.011. [DOI] [PubMed] [Google Scholar]
- NRCAN. Spruce Budworm. Available at: http://www.nrcan.gc.ca/forests/insects-diseases/13383 [Accessed June 28, 2014] [Google Scholar]
- Pavy N, Boyle B, Nelson C, et al. Identification of conserved core xylem gene sets: conifer cDNA microarray development, transcript profiling and computational analyses. New Phytol. 2008;180:766–786. doi: 10.1111/j.1469-8137.2008.02615.x. [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Poland T, McCullough D. Emerald ash borer: invasion of the urban forest and the threat to North America's ash resource. J. Forestry. 2006;104:118–124. [Google Scholar]
- Raffa KF, Powell EN, Townsend PA. Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plant defenses. Proc. Natl Acad. Sci. USA. 2013;110:2193–2198. doi: 10.1073/pnas.1216666110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raherison E, Rigault P, Caron S, Poulin P-L, Boyle B, Verta J-P, Giguere I, Bomal C, Bohlmann J, MacKay J. Transcriptome profiling in conifers and the PiceaGenExpress database show patterns of diversification within gene families and interspecific conservation in vascular gene expression. BMC Genom. 2012;13:434. doi: 10.1186/1471-2164-13-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigault P, Boyle B, Lepage P, Cooke JEK, Bousquet J, MacKay JJ. A white spruce gene catalog for conifer genome analyses. Plant Physiol. 2011;157:14–28. doi: 10.1104/pp.111.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach CR, Hall DE, Zerbe P, Bohlmann J. Plasticity and evolution of (+)-3-carene synthase and (−)-sabinene synthase functions of a Sitka spruce monoterpene synthase gene family associated with weevil resistance. J. Biol. Chem. 2014;289:23859–23869. doi: 10.1074/jbc.M114.571703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert JA, Madilao LL, White R, Yanchuk A, King J, Bohlmann J. Terpenoid metabolite profiling in Sitka spruce identifies association of dehydroabietic acid, (+)-3-carene, and terpinolene with resistance against white pine weevil. Botany. 2010;88:810–820. [Google Scholar]
- Romero IG, Ruvinsky I, Gilad Y. Comparative studies of gene expression and the evolution of gene regulation. Nat. Rev. Genet. 2012;13:505–516. doi: 10.1038/nrg3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge R, Stewart D. A kinetic-based sigmoidal model for the polymerase chain reaction and its application to high-capacity absolute quantitative real-time PCR. BMC Biotech. 2008;8:47. doi: 10.1186/1472-6750-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury F, Thuenemann EC, Lomonossoff GP. pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 2009;7:682–693. doi: 10.1111/j.1467-7652.2009.00434.x. [DOI] [PubMed] [Google Scholar]
- Sainsbury F, Varennes-Jutras P, Goulet M-C, D'Aoust M-A, Michaud D. Tomato cystatin SlCYS8 as a stabilizing fusion partner for human serpin expression in plants. Plant Biotechnol. J. 2013;11:1058–1068. doi: 10.1111/pbi.12098. [DOI] [PubMed] [Google Scholar]
- Sansenya S, Opassiri R, Kuaprasert B, Chen C-J, Cairns JRK. The crystal structure of rice (Oryza sativa L.) Os4BGlu12, an oligosaccharide and tuberonic acid glucoside-hydrolyzing β-glucosidase with significant thioglucohydrolase activity. Arch. Biochem. Biophys. 2011;510:62–72. doi: 10.1016/j.abb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Strunz GM, Giguère P, Thomas AW. Synthesis of pungenin, a foliar constituent of some spruce species, and investigation of efficacy as a feeding deterrent for spruce budworm (Choristoneura fumiferana (Clem.)) J. Chem. Ecol. 1986;12:251–260. doi: 10.1007/BF01045608. [DOI] [PubMed] [Google Scholar]
- Todesco M, Balasubramanian S, Hu TT, et al. Natural allelic variation underlying a major fitness trade-off in Arabidopsis thaliana. Nature. 2010;465:632–636. doi: 10.1038/nature09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comp. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoucq L, Moriniere J, Bevan DR, Esen A, Vasella A, Henrissat B, Czjze M. Structural determinants of substrate specificity in family 1 β-glucosidases: novel insights from the crystal structure of sorghum dhurrinase-1, a plant β-glucosidase with strict specificity, in complex with its natural substrate. J. Biol. Chem. 2004;279:31796–31803. doi: 10.1074/jbc.M402918200. [DOI] [PubMed] [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003;33:949–956. doi: 10.1046/j.1365-313x.2003.01676.x. [DOI] [PubMed] [Google Scholar]
- Wray GA. The evolutionary significance of cis-regulatory mutations. Nat. Rev. Genet. 2007;8:206–216. doi: 10.1038/nrg2063. [DOI] [PubMed] [Google Scholar]
- Yanchuk A, Murphy J, Wallin K. Evaluation of genetic variation of attack and resistance in lodgepole pine in the early stages of a mountain pine beetle outbreak. Tree Genet. Genomes. 2008;4:171–180. [Google Scholar]
- Züst T, Heichinger C, Grossniklaus U, Harrington R, Kliebenstein DJ, Turnbull LA. Natural enemies drive geographic variation in plant defenses. Science. 2012;338:116–119. doi: 10.1126/science.1226397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of RT-quantitative PCR assay.
Predicted amino acid sequence of PgβGLU-1 protein.
Differentially expressed genes between resistant (R) and non-resistant (N-R) trees.
Variations in Pgβglu-1 amino acid sequence of resistant (R) and non-resistant (N-R) trees.
White spruce provenances sampled for the population survey.
Primer sequences used for quantitative PCR analysis, molecular cloning, and sequencing assays.


