Figure 4.
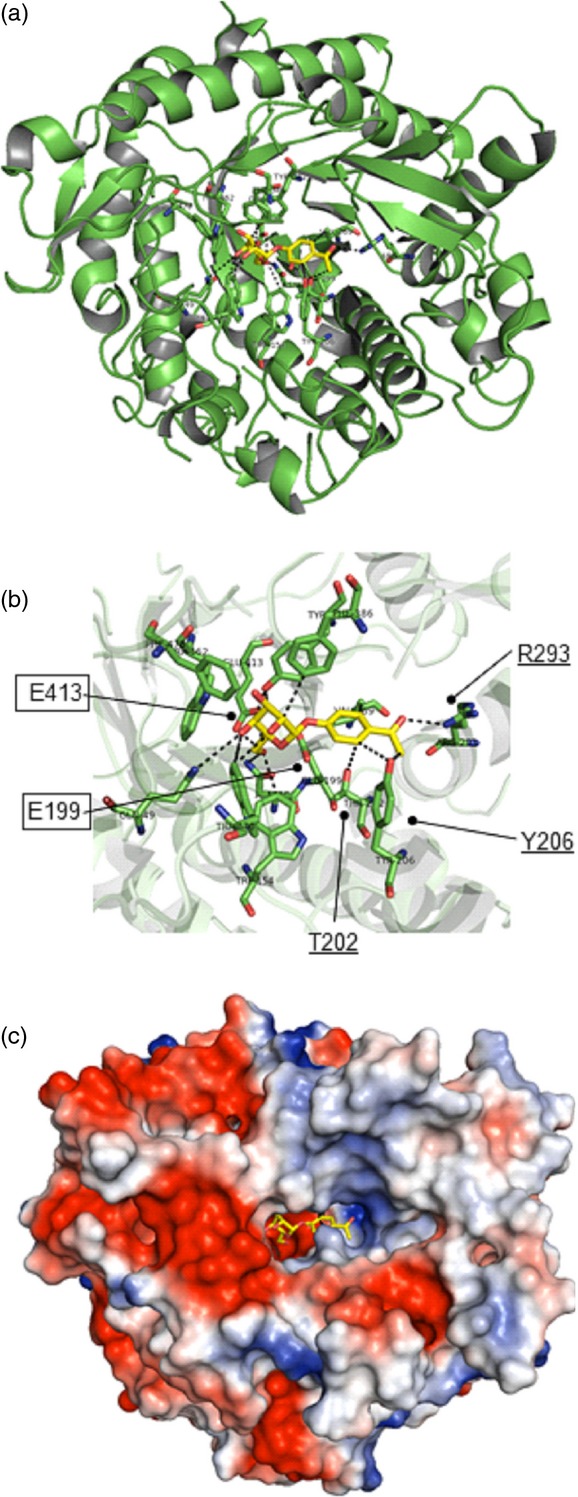
Structural model of the Pgβglu-1 gene product.(a) Cartoon representation of the overall structure of the PgβGLU-1 protein.(b) Predicted PgβGLU-1 Michaelis complex with picein. Catalytic site glutamic acid residues (boxed, E199, E413): nucleophile E413 Oe1 was hydrogen bonded (dashed lines) with O5 (2.92 Å) and the distance between Oe2 of the catalytic acid/base residue E199 and O1 of picein is 3.35 Å. Three amino acids (underlined, T202, Y206, R293) predicted to form hydrogen bonds with the phenolic moiety of picein.(c) Electrostatic potential surface representation, negatively and positively charged areas in red and blue, respectively.
