Abstract
Objectives
The objective was to assess adverse outcomes in relation to the simplified Pulmonary Embolism Severity Index (PESI) score in patients treated with rivaroxaban or standard therapy in the phase III EINSTEIN PE study and to evaluate the utility of the simplified PESI score to identify low-risk pulmonary embolism (PE) patients.
Methods
A post hoc analysis of EINSTEIN PE data was performed to assess the efficacy and safety of rivaroxaban in patients with a range of simplified PESI scores. Recurrent venous thromboembolism, fatal PE, all-cause mortality, and major bleeding were stratified by simplified PESI scores of 0, 1, or ≥2 and according to treatment period at 7, 14, 30, and 90 days and at the end of the full intended treatment period.
Results
Simplified PESI scores could be calculated in 4,831 of the 4,832 randomized patients; of those, 53.6, 36.7, and 9.7% had PESI scores of 0, 1, and ≥2, respectively. Among patients with simplified PESI scores of 0 or 1, fatal PE, all-cause mortality, and other adverse outcomes were uncommon within the first 7, 14, and 30 days. Patients with simplified PESI scores of ≥2 had more frequent adverse outcomes. Major bleeding was lower in the rivaroxaban group, particularly in those with simplified PESI scores of 1 or ≥2.
Conclusions
The findings support using risk stratification with the simplified PESI score to identify low-risk patients with PE.
Venous thromboembolism (VTE) comprises deep vein thrombosis (DVT) and pulmonary embolism (PE). Acute PE is a potentially life-threatening medical emergency that requires urgent intervention. In the United States, most patients with PE are admitted to the hospital, whereas patients with acute DVT are often managed as outpatients with coordinated care through a primary provider. Current standard therapy for most patients with acute PE or DVT is the same: anticoagulation therapy with a parenteral agent (e.g., subcutaneous administration of low-molecular-weight heparin) overlapping with and followed by a vitamin K antagonist (VKA).1,2
The Pulmonary Embolism Severity Index (PESI) and simplified PESI are extensively validated prognostic tools for predicting death and adverse outcome events in patients with PE.3–5 The simplified PESI score has proven useful to physicians and allows risk stratification of patients with PE. Patients found to be at low risk using this measure may be able to be discharged safely from the emergency department (ED).4,5 The standard dual-drug anticoagulation therapy is effective but complex. Non-VKA direct oral anticoagulants, given as fixed-dose regimens, have been shown to have similar efficacy and safety profiles to standard therapy in phase III studies. The advantage of these agents is their potential to improve patients' quality of life by simplifying patient management and offering the therapeutic flexibility of oral administration without the need for routine coagulation monitoring (a limitation of the VKAs). Results from the phase III EINSTEIN PE study showed that rivaroxaban was noninferior to enoxaparin/VKA for the efficacy endpoint of recurrent VTE and the safety outcome of major and clinically relevant nonmajor bleeding.6,7 Given the similar safety profile of rivaroxaban to enoxaparin/VKA therapy, the identification of a cohort of PE patients (e.g., those with low simplified PESI scores) with very few short-term (within 7 to 14 days) adverse events could support the consideration of direct discharge from the ED. Therefore, the results of this analysis provide a framework for an informed discussion with the patient regarding his or her prognosis and management pathway.
This post hoc analysis assessed adverse outcomes in relation to the simplified PESI score in patients with symptomatic PE who were treated with rivaroxaban or standard therapy in the phase III EINSTEIN PE study and evaluated the use of the simplified PESI score to identify low-risk patients with acute PE who could be considered for treatment with rivaroxaban or standard therapy out of the hospital setting.
Methods
Study Design
The EINSTEIN PE study was an open-label, randomized, phase III study (trial registration number NCT00439777) comparing oral rivaroxaban (15 mg twice daily for 3 weeks, followed by 20 mg once daily thereafter) with subcutaneous enoxaparin overlapping with and followed by a VKA (warfarin or acenocoumarol; target international normalized ratio = 2.0 to 3.0) for 3, 6, or 12 months. The study protocol was approved by the institutional review board at each center, and written informed consent was obtained from all patients.6
The EINSTEIN DVT and EINSTEIN PE studies were supported by Bayer HealthCare Pharmaceuticals and Janssen Research & Development, LLC, who gathered, maintained, and extracted data. The authors had access to the data and were responsible for interpreting the data and writing the article. The corresponding author had the final responsibility to submit this article for publication.
Study Setting and Population
Patients who had acute symptomatic PE with or without DVT were enrolled from 38 countries. The diagnosis of PE was based on one of the following: an intraluminal filling defect in segmental or more proximal branches on spiral computed tomography; an intraluminal filling defect or a sudden cutoff of vessels more than 2.5 mm in diameter on pulmonary angiogram; a perfusion defect of at least 75% of a segment with a local normal ventilation result (high probability) on ventilation/perfusion lung scintigraphy; or inconclusive spiral computed tomography, pulmonary angiography, or lung scintigraphy, with demonstration of DVT in the lower extremities by compression ultrasound or venography.
Simplified PESI Score
Using baseline data from the EINSTEIN PE study, one point was assigned for each of the following patient characteristics: age > 80 years, history of cancer, chronic cardiopulmonary disease, pulse ≥ 110 beats/minute, systolic blood pressure (sBP) < 100 mm Hg, and arterial oxyhemoglobin saturation < 90%, according to the simplified PESI score (Table1). Patients with none of the above variables were classified as low risk (i.e., PESI score 0).
Table 1.
Simplified Pulmonary Embolism Severity Index
| Variable | Simplified PESI score |
|---|---|
| Age > 80 yr | 1 |
| History of cancer | 1 |
| Chronic cardiopulmonary disease* | 1 |
| Pulse ≥ 110 beats/min | 1 |
| sBP < 100 mm Hg | 1 |
| Arterial oxyhemoglobin saturation level < 90% | 1 |
Combined variable of history of heart failure and history of chronic lung disease.
Outcome Measures
Outcomes were analyzed according to the simplified PESI score and included recurrent VTE, fatal PE, major bleeding, and all-cause mortality at 7, 14, 30, and 90 days and at the end of the full intended treatment period. Recurrent VTE was defined as a composite of DVT and nonfatal or fatal PE.6 Bleeding was defined as major if it was clinically overt and was associated with a decrease in hemoglobin level of ≥2.0 g/dL, led to the transfusion of ≥2 units of red blood cells, was intracranial or retroperitoneal or occurred in another critical site, or contributed to death.6 PE was considered the cause of death if there was objective documentation of the condition, or if death could not be attributed to a documented cause and PE could not be confidently ruled out.
Data Analysis
This post hoc analysis was performed to assess the efficacy and safety of rivaroxaban in patients with a range of simplified PESI scores from the EINSTEIN PE study. Recurrent VTE, fatal PE, all-cause mortality, and major bleeding were related to the simplified PESI score and data were stratified by simplified PESI scores of 0, 1, or ≥2 and according to treatment period. Clinical outcomes are presented as percentage incidences, together with odds ratios (ORs) and 95% confidence intervals (CIs) for the factors (treatment group and PESI scores) estimated using direct logistic regression models. Associations between simplified PESI scores and clinical outcomes were also assessed and p-values are presented (Wald chi-square test for the factor effect) from the corresponding direct logistic regression models. Given the post hoc nature of the current analysis, the p-values presented are unadjusted and significance of the values should be interpreted with caution. All analyses were done with SAS version 9.2
Results
A total of 4,832 patients were randomized in the EINSTEIN PE study. Patient demographics, including age, sex, and creatinine clearance, were similar between the rivaroxaban and standard therapy treatment groups (Table2). More than half of the patients (57 and 58% for the rivaroxaban and standard therapy groups, respectively) received prerandomization treatment with parenteral anticoagulants (low-molecular-weight heparin, heparin, or fondaparinux) for 1 day; approximately one-third of the patients received 2 days of prerandomization treatment. Similar proportions of patients in the two treatment groups had risk factors associated with VTE, but a slightly higher percentage of patients in the rivaroxaban group had a history of heart failure and ischemic heart disease. Six-hundred patients (12.4%) in EINSTEIN PE were admitted to intensive care units, 3,719 (77.0%) were treated in non–intensive care unit hospital settings, and 513 (10.6%) were not hospitalized (Table2).
Table 2.
Demographics and Clinical Characteristics of Patients
| Characteristic | Rivaroxaban (n = 2,419) | Standard Therapy* (n = 2,413) |
|---|---|---|
| Age (yr), mean ± SD | 57.9 ± 17.3 | 57.5 ± 17.2 |
| Male sex | 1,309 (54.1) | 1,247 (51.7) |
| Creatinine clearance (mL/min) | ||
| <30 | 4 (0.2) | 2 (<0.1) |
| 30–49 | 207 (8.6) | 191 (7.9) |
| 50–79 | 637 (26.3) | 593 (24.6) |
| ≥80 | 1,555 (64.3) | 1,617 (67.0) |
| Missing | 16 (0.7) | 10 (0.4) |
| Risk factor associated with VTE | ||
| Recent surgery or trauma | 415 (17.2) | 398 (16.5) |
| Previous VTE | 455 (18.8) | 489 (20.3) |
| Active cancer | 114 (4.7) | 109 (4.5) |
| Estrogen therapy | 207 (8.6) | 223 (9.2) |
| Immobilization | 384 (15.9) | 380 (15.7) |
| Known thrombophilic condition | 138 (5.7) | 121 (5.0) |
| Unprovoked VTE | 1,566 (64.7) | 1,551 (64.3) |
| Comorbidity | ||
| Heart failure | 62 (2.6) | 45 (1.9) |
| COPD or asthma | 287 (11.9) | 280 (11.6) |
| Ischemic heart disease | 226 (9.3) | 181 (7.5) |
| Stroke | 113 (4.7) | 116 (4.8) |
| Clinical presentation | ||
| Pulse > 100 beats/min | 193 (8.0) | 195 (8.1) |
| sBP < 100 mm Hg | 56 (2.3) | 64 (2.7) |
| Arterial oxygen saturation | ||
| <90% | 52 (2.1) | 66 (2.7) |
| ≥90% to <95% | 526 (21.7) | 488 (20.2) |
| ≥95% | 1,745 (72.1) | 1,759 (72.9) |
| Missing | 96 (4.0) | 100 (4.1) |
| Level of care | ||
| ICU | 311 (12.9) | 289 (12.0) |
| Non-ICU | 1,847 (76.4) | 1,872 (77.6) |
| Not hospitalized | 261 (10.8) | 252 (10.4) |
Data are n (%) unless otherwise noted.
COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; VKA = vitamin K antagonist; VTE = venous thromboembolism.
Standard treatment consisted of subcutaneous enoxaparin overlapping with and followed by a VKA (warfarin or acenocoumarol; target international normalized ratio = 2.0 to 3.0).
Of the 4,832 patients randomized in EINSTEIN PE, the simplified PESI score could be calculated for 4,831 patients. Among these, 2,589 (53.6%) patients had scores of 0; 1,775 (36.7%) had scores of 1; and 467 (9.7%) had scores of ≥2. No patient had a simplified PESI score higher than 3. Values of pulse rate, sBP, and arterial oxyhemoglobin saturation level used for calculating PESI scores were taken at the screening visit prior to randomization.
There was a significant association between simplified PESI score and almost all of the major adverse outcomes (recurrent VTE, fatal PE, all-cause mortality, and major bleeding), with high PESI score corresponding to increased risk of the adverse clinical outcome (Figure1; Data Supplement S1, available as supporting information in the online version of this paper). For example, the simplified PESI score was significantly associated with fatal PE and all-cause mortality up to 7 days (p = 0.014 and p < 0.0001, respectively) and up to 14 days (p = 0.035 and p < 0.0001, respectively) and up to the end of the intended treatment period (p = 0.0001 and p < 0.0001).
Figure 1.
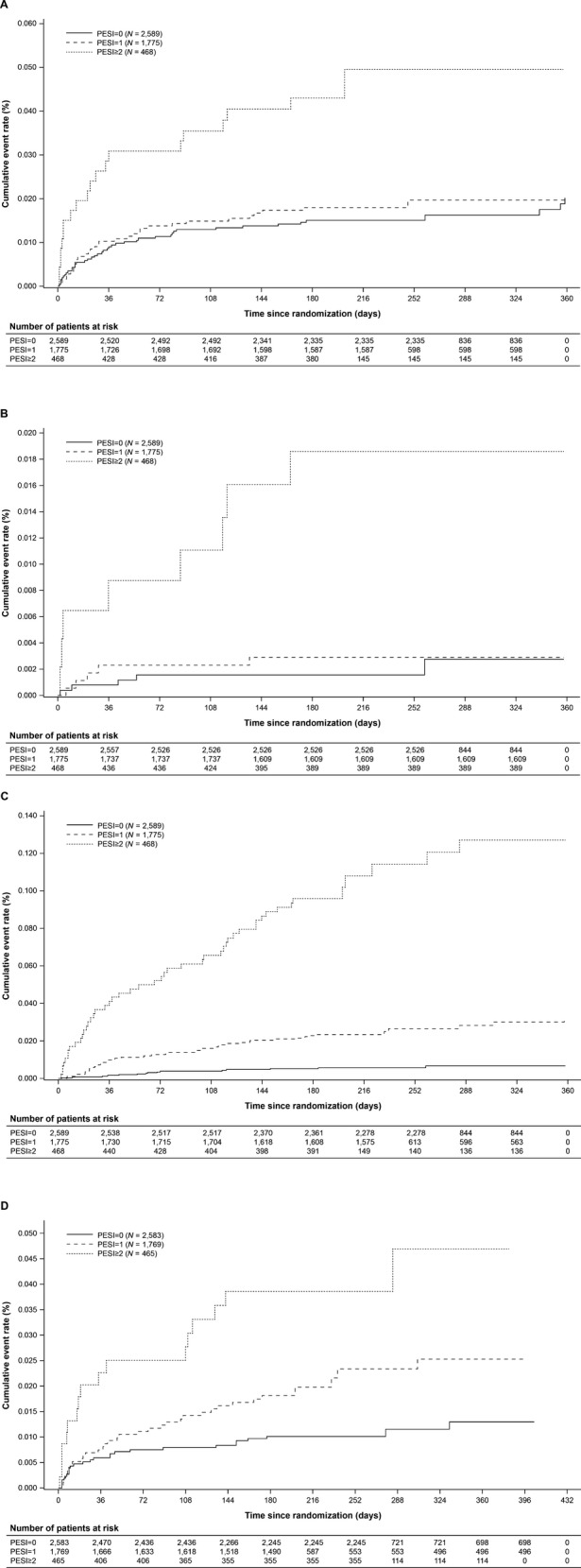
Cumulative incidences of recurrent VTE (A), fatal PE (B), all-cause mortality up to the end of intended treatment period (intention-to-treat population; C), and treatment-emergent major bleeding events (safety population; D). PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; VTE = venous thromboembolism.
In patients with simplified PESI scores of 0 or 1, the incidences of major adverse outcomes were low during the first 30 days of treatment. For example, there were no cases of fatal PE up to Day 7 among rivaroxaban-treated patients; the incidences were <0.1 and 0.1% for patients in the standard therapy group who had simplified PESI scores of 0 or 1, respectively. Patients with simplified PESI scores of 0 had a recurrent VTE rate of 0.8% (10 of the 1,256) with rivaroxaban and 0.7% (9 of 1,333) with standard therapy within 30 days, and the incidence was 1.0% (9 of 919) and 1.1% (9 of 856), respectively, for those with simplified PESI scores of 1. All-cause mortality rate was low and similar in patients with simplified PESI scores of 0 between 30 and 7 or 14 days, but the incidence was higher in patients with simplified PESI scores of 1 at 30 days compared with that at 7 or 14 days (Data Supplement S2, available as supporting information in the online version of this paper).
Patients with simplified PESI scores of ≥2 had more frequent events of recurrent VTE, fatal PE, all-cause mortality, and major bleeding than patients with simplified PESI scores of 0 or 1 at all measured time points. The incidences were also numerically higher at 90 days compared with those at 7, 14, and 30 days in patients with simplified PESI scores of ≥2 in both treatment groups (Data Supplement S2).
Higher simplified PESI scores were associated with higher incidences of major bleeding and all-cause mortality; the cumulative incidences of these events according to the simplified PESI are shown in Figures1C and 1D. In patients with simplified PESI scores of 0, the incidences of major bleeding events were broadly similar between the two treatment groups at the different treatment periods; for the full treatment period, major bleeding occurred in 11 of the 1,254 patients (0.9%) in the rivaroxaban group and 16 of the 1,329 patients (1.2%) in the standard therapy group. In patients with simplified PESI scores of 1, major bleeding occurred in 1.1% of rivaroxaban-treated patients and in 2.8% of standard therapy-treated patients; the incidences were 2.1 and 5.4% for rivaroxaban and standard therapy, respectively, in patients with simplified PESI scores of ≥2 (full treatment period; Data Supplement S2). Rates of all-cause mortality were low and similar in the rivaroxaban and standard therapy groups in patients with simplified PESI scores of ≤1 up to days 7, 14, and 30 of treatment. Results at 90 days showed increased rates of all-cause mortality in patients with simplified PESI scores of ≥1 in both treatment groups (Data Supplement S2).
Data from further analyses of adverse outcomes in relation to simplified PESI score and hospitalization for the index event are shown in Table3. Because the number of subjects with PE who were treated as outpatients was low, the findings are considered exploratory. In patients with simplified PESI scores of ≤1 who were treated as outpatients, there were no cases of fatal PE or all-cause mortality up to 14 days (for a score of 0 or 1), and up to 30 days (for a score of 0; Table3). However, a PESI of 0 had a diagnostic sensitivity for recurrent VTE of 12 of 21 (57%, 95% CI = 34% to 78%) and major bleeding of 10 of 17 (59%, 95% CI = 33% to 82%) at ≤7 days. The incidence of major bleeding events in patients with simplified PESI scores of ≤1 was similar or numerically higher in outpatients compared with inpatients at up to 30 days; however, in patients with simplified PESI scores of ≥2 who were treated as outpatients, there were no major bleeding events (Table3).
Table 3.
Any Adverse Outcomes From the EINSTEIN PE Study in Relation to Simplified PESI Score and Hospitalization for the Index Event
| Hospitalization for the Index Event | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Window | PESI Score | Recurrent VTE* | Fatal PE* | All-cause Mortality* | Major Bleeding† | ||||
| Yes | No | Yes | No | Yes | No | Yes | No | ||
| ≤7 days | 0 | 6/2,299 (0.3) | 3/290 (1.0) | 1/2,299 (<0.1) | 0/290 (0.0) | 1/2,299 (<0.1) | 0/290 (0.0) | 5/2,294 (0.2) | 2/289 (0.7) |
| 1 | 4/1,597 (0.3) | 1/178 (0.6) | 1/1,597 (<0.1) | 0/178 (0.0) | 2/1,597 (0.1) | 0/178 (0.0) | 4/1,592 (0.3) | 1/177 (0.6) | |
| ≥2 | 6/423 (1.4) | 1/45 (2.2) | 2/423 (0.5) | 1/45 (2.2) | 4/423 (0.9) | 3/45 (6.7) | 5/421 (1.2) | 0/44 (0.0) | |
| ≤14 days | 0 | 11/2,299 (0.5) | 3/290 (1.0) | 2/2,299 (<0.1) | 0/290 (0.0) | 2/2,299 (<0.1) | 0/290 (0.0) | 10/2,294 (0.4) | 2/289 (0.7) |
| 1 | 11/1,597 (0.7) | 1/178 (0.6) | 2/1,597 (0.1) | 0/178 (0.0) | 4/1,597 (0.3) | 0/178 (0.0) | 8/1,592 (0.5) | 1/177 (0.6) | |
| ≥2 | 7/423 (1.7) | 2/45 (4.4) | 2/423 (0.5) | 1/45 (2.2) | 6/423 (1.4) | 3/45 (6.7) | 6/421 (1.4) | 0/44 (0.0) | |
| ≤30 days | 0 | 16/2,299 (0.7) | 3/290 (1.0) | 2/2,299 (<0.1) | 0/290 (0.0) | 2/2,299 (<0.1) | 0/290 (0.0) | 13/2,294 (0.6) | 2/289 (0.7) |
| 1 | 15/1,597 (0.9) | 3/178 (1.7) | 3/1,597 (0.2) | 1/178 (0.6) | 12/1,597 (0.8) | 2/178 (1.1) | 10/1,592 (0.6) | 2/177 (1.1) | |
| ≥2 | 10/423 (2.4) | 2/45 (4.4) | 2/423 (0.5) | 1/45 (2.2) | 14/423 (3.3) | 3/45 (6.7) | 9/421 (2.1) | 0/44 (0.0) | |
| Full treatment period | 0 | 35/2,299 (1.5) | 7/290 (2.4) | 5/2,299 (0.2) | 0/290 (0.0) | 14/2,299 (0.6) | 1/290 (0.3) | 24/2,294 (1.0) | 3/289 (1.0) |
| 1 | 29/1,597 (1.8) | 3/178 (1.7) | 4/1,597 (0.3) | 1/178 (0.6) | 38/1,597 (2.4) | 7/178 (3.9) | 29/1,592 (1.8) | 5/177 (2.8) | |
| ≥2 | 18/423 (4.3) | 2/45 (4.4) | 7/423 (1.7) | 1/45 (2.2) | 43/423 (10.2) | 5/45 (11.1) | 17/421 (4.0) | 0/44 (0.0) | |
Data are reported as n/N (%).
PE = pulmonary embolism; PESI = Pulmonary Embolism Severity Index; VTE = venous thromboembolism.
Intention-to-treat population.
Safety population; Major bleeding defined according to the Control of Anticoagulation Subcommittee (ISTH SSC 2004): fatal bleeding and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome; bleeding causing a fall in hemoglobin level of 20 g/L (1.24 mmol/L) or more or leading to transfusion of ≥2 units of whole blood or red cells21 (data derived from the safety population).
Discussion
The PESI and simplified PESI are prognostic assessment tools for the management of patients with acute PE that have been proposed as tools to select patients for outpatient therapy. The accuracy of these two scoring systems has been assessed and compared in a meta-analysis8 and the results indicate that the PESI has discriminative power to predict death and adverse outcome events in patients with acute PE. The PESI (consisting of 11 variables) and the simplified PESI (consisting of six of the 11 variables in the PESI) have similar accuracy, but the simplified PESI is easier to use.8 Most of the studies in the meta-analysis had follow-up periods of 30 days (although in some studies this was 3 or 6 months, or in hospital) but the most relevant data come from the 7- and 14-day outcomes because decisions to treat in the outpatient setting will not be influenced by 30-day outcomes. The results of the meta-analysis showed that low-risk PESI scores were significantly associated with lower all-cause mortality, lower PE-related mortality, and fewer serious adverse events.8 The pooled all-cause mortality was 1.8% in patients with low-risk simplified PESI scores, compared with 25.2% in patients with high-risk simplified PESI scores. The incidence of PE-related mortality was 0.4% in the low-risk patients and 5.1% in the high-risk patients, based on the simplified PESI.8 In this post hoc analysis of the EINSTEIN PE study, the all-cause mortality rates were notably lower than those reported in the high-risk patients in the meta-analysis.8 Similarly, the incidences of fatal PE were lower in this post hoc analysis than those reported in the meta-analysis in both low- and high-risk patient groups. Given the differences between the PESI meta-analysis and this EINSTEIN PE post hoc analysis, a closer examination of the risk profile of the study population is warranted.
A valid consideration in evaluating the outcomes of the EINSTEIN PE data set is whether or not the study cohort is representative of the overall population of ED patients presenting for evaluation for VTE. Several registries have described the demographics, diagnostic evaluation, treatment, and outcomes of acute PE.9–11 The age and sex of patients in the EINSTEIN PE group resembles those enrolled in the EMPEROR (Emergency Medicine Pulmonary Embolism in the Real World Registry9), registry of patients presenting to EDs in the United States.12 With respect to mortality, the EINSTEIN PE data set resembles the EMPEROR cohort rather than those of the ICOPER (International Cooperative Pulmonary Embolism Registry10) or RIETE (Registro Informatizado de la Enfermedad ThromboEmbolica11) registries. In EMPEROR, the PE-attributed and all-cause mortality rates at 30 days were 1.1 and 5.4%, respectively.12 In EINSTEIN PE, the overall incidences of PE-related deaths and all-cause mortality up to 30 days were 0.2 and 0.7%, respectively; in patients with simplified PESI scores of ≥2, these were 0.6 and 3.6%, respectively. In the EINSTEIN PE analysis, 2.3% to 2.7% of patients were hypotensive (sBP < 100 mm Hg) and 2.7% to 2.9% were hypoxic (arterial oxygen saturation < 90%), whereas in EMPEROR, 3.0% of the cohort were hypotensive (sBP < 90 mm Hg).12 Conversely, the ICOPER registry enrolled a higher risk, inpatient cohort of patients whose 14-day mortality rate was 11.2%.9 The RIETE group reported a 3.3% rate of 30-day PE-related mortality.11 Given the available registry data on patients presenting to EDs in the United States, EINSTEIN PE appears to have enrolled patients with marginally lower risk profiles than those enrolled in EMPEROR and much lower risk profiles than those enrolled in ICOPER and RIETE.
This analysis showed that there was a close relationship between the major clinical outcomes (recurrent VTE, fatal PE, major bleeding, and all-cause mortality) and the simplified PESI score of the patients. Patients with simplified PESI scores of 0 or 1 experienced fewer major clinical outcome events during the first 30 days of treatment (i.e., based on data up to days 7, 14, and 30) in comparison with those patients with simplified PESI scores of ≥2. Patients with simplified PESI scores of ≥2 experienced more recurrent VTE, fatal PE, and major bleeding complications than those with scores of <2 in all treatment periods measured, and these results were similar in patients treated with rivaroxaban or standard therapy. Data up to 30 days showed that in patients with simplified PESI scores of 1, the rate of major bleeding was 0.3% in the rivaroxaban group and 1.1% in the standard therapy group; in those with simplified PESI scores of ≥2, major bleeding occurred in 0.8 and 3.2% of patients in the rivaroxaban and the standard therapy groups, respectively.
Although guidelines, such as those from the American College of Chest Physicians, recommend outpatient treatment for selected PE patients at low risk of recurrence,2 existing evidence for the outpatient management of patients with PE is derived from small cohorts of patients from outside the United States.13–16 The results of this analysis provide further support that risk stratification of PE patients may allow a cohort of low-risk patients to be treated in a clinical decision unit or by a closely monitored outpatient strategy. Such an approach might relieve some of the burden placed on the ED. Because the incidences of major clinical events, such as fatal PE and major bleeding, are relatively low in the first 30 days of therapy, emergency clinicians may consider offering these low-risk patients an alternative to the traditional inpatient stay. This is supported by the finding of this analysis that in patients with simplified PESI scores of ≤1 who were treated as outpatients, there were no cases of fatal PE or all-cause mortality up to 14 days (for a score of 0 or 1) and up to 30 days (for a score of 0). Conversely, the data are also consistent with previous studies in which patients classified as being at high risk of VTE recurrence and 30-day mortality had higher rates of recurrent VTE, bleeding, and overall mortality than low-risk patients.5,17,18 Furthermore, although no statistical comparison between the two treatment groups for the major clinical outcomes could be made (owing to the low event rates and the small number of patients per subgroup), data from the current analysis suggest that rivaroxaban may provide an equally effective treatment option to standard therapy for both low- and high-risk patients, with a potentially lower risk of major bleeding complications.
Limitations
Owing to the nature of a post hoc study, any significant values must be interpreted with caution. In the current analysis, no multiple testing was conducted and p-values remain unadjusted. Moreover, a selection bias arising from the randomized open-label design of the original EINSTEIN PE study cannot be ruled out. In addition to examining the study population, the risk stratification tool used in this post hoc investigation can be scrutinized. The simplified PESI score was chosen for use in this analysis in preference to the original PESI and HESTIA schemes (consisting of 11 variables) because of the clinical applicability of having only six variables that are easily assessed early in the acute care evaluation of PE patients.16,19 Although the PESI and simplified PESI are powerful predictors of short-term mortality, intangible social factors that may mandate inpatient therapy are not part of the model. The HESTIA scheme includes not only physiological parameters, but also sociobehavioral factors that optimize its effectiveness as a decision tool. However, the EINSTEIN database is unable to recover the social factors that would be necessary to evaluate the HESTIA criteria accurately. Additionally, the PESI criteria have been described as a sound tool for predicting mortality; however, they lack the ability to predict patients at low risk of VTE complications. Accordingly, this investigation confirms that a PESI score of 0 has limited utility in the prediction of both recurrent VTE and major bleeding events at ≤7 days. Although it is tempting to conclude that risk stratification using only short-term mortality would allow for safe discharge, clinicians are acutely aware that socioeconomic factors complicate the disposition. Despite the fact that the simplified PESI criterion has been extensively validated as a risk stratification instrument, the individual components of the score may perform differently in prediction of 5-to 7-day versus 30-day outcomes. Some authors have suggested that a model to predict in-hospital or 5-to 7-day clinical deterioration would be more relevant to the clinical decision to admit or discharge a patient with PE than a 30-day outcome.20
Another limitation of the PESI and simplified PESI scores is that they were not designed to incorporate bleeding events. In addition, the current analysis does not address any potential influence of renal function (e.g., creatinine clearance rate) on the outcomes, nor provide any information with respect to the treatment of subsegmental PE.
Conclusions
This post hoc analysis of the phase III EINSTEIN PE study demonstrated that risk stratification using the simplified Pulmonary Embolism Severity Index score could identify low-risk patients with newly diagnosed pulmonary embolism and suggests that rivaroxaban may provide an effective alternative treatment option to standard therapy for low- and high-risk pulmonary embolism patients, with reduced risk of major bleeding complications.
The authors acknowledge Yong-Ling Liu, who provided editorial assistance with funding from Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC.
Supporting Information
Data Supplement S1. Association of Pulmonary Embolism Severity Index (PESI) scores with outcomes. Estimates of main treatment effects by time period using logistic regression models.
Data Supplement S2. Any adverse outcomes from the EINSTEIN PE study in relation to simplified Pulmonary Embolism Severity Index score and time period: (A) ≤7 days; (B) ≤14 days; (C) ≤30 days; (D) ≤90 days; and (E) full treatment period.
References
- Torbicki A, Perrier A, Konstantinides S. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujesky D, Obrosky DS, Stone RA. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–6. doi: 10.1164/rccm.200506-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez D, Aujesky D, Díaz G. Prognostic significance of deep vein thrombosis in patients presenting with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2010;181:983–91. doi: 10.1164/rccm.200908-1204OC. [DOI] [PubMed] [Google Scholar]
- Fraga M, Taffe P, Mean M. The inter-rater reliability of the Pulmonary Embolism Severity Index. Thromb Haemost. 2010;104:1258–62. doi: 10.1160/TH10-07-0426. [DOI] [PubMed] [Google Scholar]
- The EINSTEIN-PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- Prins MH, Lensing AW. Derivation of the non-inferiority margin for the evaluation of direct oral anticoagulants in the treatment of venous thromboembolism. Thromb J. 2013;11:13. doi: 10.1186/1477-9560-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XY, Ben SQ, Chen HL, Ni SS. The prognostic value of pulmonary embolism severity index in acute pulmonary embolism: a meta-analysis. Respir Res. 2012;13:111. doi: 10.1186/1465-9921-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–9. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- Kasper W, Konstantinides S, Geibel A. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165–71. doi: 10.1016/s0735-1097(97)00319-7. [DOI] [PubMed] [Google Scholar]
- Laporte S, Mismetti P, Décousus H. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117:1711–6. doi: 10.1161/CIRCULATIONAHA.107.726232. [DOI] [PubMed] [Google Scholar]
- Pollack CV, Schreiber D, Goldhaber SZ. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department initial report of EMPEROR (multicenter emergency medicine pulmonary embolism in the real world registry) J Am Coll Cardiol. 2011;57:700–6. doi: 10.1016/j.jacc.2010.05.071. [DOI] [PubMed] [Google Scholar]
- Otero R, Uresandi F, Jimenez D. Home treatment in pulmonary embolism. Thromb Res. 2010;126:e1–5. doi: 10.1016/j.thromres.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Zondag W, Kooiman J, Klok FA, Dekkers OM, Huisman MV. Outpatient versus inpatient treatment in patients with pulmonary embolism: a meta-analysis. Eur Respir J. 2013;42:134–44. doi: 10.1183/09031936.00093712. [DOI] [PubMed] [Google Scholar]
- Aujesky D, Roy PM, Verschuren F. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378:41–8. doi: 10.1016/S0140-6736(11)60824-6. [DOI] [PubMed] [Google Scholar]
- Zondag W, Mos IC, Creemers-Schild D. Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost. 2011;9:1500–7. doi: 10.1111/j.1538-7836.2011.04388.x. [DOI] [PubMed] [Google Scholar]
- Erkens PM, Gandara E, Wells PS. Does the Pulmonary Embolism Severity Index accurately identify low risk patients eligible for outpatient treatment? Thromb Res. 2012;129:710–4. doi: 10.1016/j.thromres.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Aujesky D, Roy PM, Le Manach CP. Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur Heart J. 2006;27:476–81. doi: 10.1093/eurheartj/ehi588. [DOI] [PubMed] [Google Scholar]
- Aujesky D, Perrier A, Roy PM. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med. 2007;261:597–604. doi: 10.1111/j.1365-2796.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- Kabrhel C, Okechukwu I, Hariharan P. Factors associated with clinical deterioration shortly after PE. Thorax. 2014;69:835–42. doi: 10.1136/thoraxjnl-2013-204762. [DOI] [PubMed] [Google Scholar]
- Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S1. Association of Pulmonary Embolism Severity Index (PESI) scores with outcomes. Estimates of main treatment effects by time period using logistic regression models.
Data Supplement S2. Any adverse outcomes from the EINSTEIN PE study in relation to simplified Pulmonary Embolism Severity Index score and time period: (A) ≤7 days; (B) ≤14 days; (C) ≤30 days; (D) ≤90 days; and (E) full treatment period.


