Abstract
Objectives
To further study the interplay between smoking status, Coronary Artery Calcium (CAC) and all-cause mortality.
Background
Prior studies have not directly compared the relative prognostic impact of CAC in smokers versus non-smokers. In particular, while zero CAC is a known favorable prognostic-marker, whether smokers without CAC have as good a prognosis as non-smokers without CAC is unknown. Given computed tomography (CT) screening for lung cancer appears effective in smokers, the relative prognostic implications of visualizing any CAC versus no CAC on such screening also deserve study.
Methods
Our study cohort consisted of 44,042 asymptomatic individuals referred for non-contrast cardiac CT (age 54±11 years, 54% males). Subjects were followed for a mean of 5.6 years. The primary endpoint was all-cause mortality.
Results
Approximately 14% (n=6020) of subjects were active smokers at enrollment. There were 901 deaths (2.05%) overall, with increased mortality in smokers vs. non-smokers (4.3% vs. 1.7%, p<0.0001). Smoking remained a risk factor for mortality across increasing strata of CAC scores (1-100, 101-400, and >400). In multivariable analysis within these strata, we found mortality hazard ratios (HRs) of 3.8 (95% CI, 2.8-5.2), 3.5 (2.6-4.9), and 2.7 (2.1-3.5), respectively, in smokers compared to nonsmokers. At each stratum of elevated CAC score, mortality in smokers was consistently higher than mortality in non-smokers from the CAC stratum above. However, among the 19,898 individuals with CAC=0, the mortality HR for smokers without CAC was 3.6 (95% CI, 2.3-5.7), compared to non-smokers without CAC.
Conclusion
Smoking is a risk factor for death across the entire spectrum of subclinical coronary atherosclerosis. Smokers with any coronary calcification are at significantly increased future mortality risk than smokers without CAC. However, the absence of CAC may not be as useful a “negative risk factor” in active smokers; as this group has mortality rates similar to non-smokers with mild to moderate atherosclerosis.
Keywords: Smoking, Subclinical Atherosclerosis, Coronary artery calcification, Cardiac CT, Prognosis
Introduction
Smoking is a leading contributor to cardiovascular disease worldwide. Among Americans greater than 18 years of age, 23% of men and 18% of women smoke1. While public health laws have helped curb smoking rates in Western populations 2, tobacco companies continue to aggressively advertise in Third World countries. Consequently, an estimated 1.3 billion people now smoke worldwide1. Smokers have increased all-cause mortality and it is estimated that up to 40% of this mortality is attributable to cardiovascular events 3.
Smoking exerts numerous pathological effects, including increases in endothelial dysfunction4, platelet reactivity5, and systemic markers of inflammation (including C-reactive Protein) 6. These aberrations accelerate the development of both clinical and subclinical atherosclerosis in smokers7. For instance, smoking has been has been strongly associated with both baseline coronary artery calcification (CAC)8 and CAC progression9-10, as measured by cardiac computed tomography (CT).
However, the interaction between smoking and CAC has not been fully elucidated. While it is known that smoking remains an independent risk factor for mortality after accounting for subclinical coronary atherosclerosis8, a number of important questions remain. Is CAC a similarly good predictor of mortality amongst both non-smokers and smokers? Prior studies have not specifically addressed this issue8. Similarly, what are the relative risks of increasing levels of baseline CAC in smokers as compared to non-smokers? Also, is mortality in smokers with CAC scores of zero higher than has been reported in the general population with CAC=011?
With new evidence demonstrating a prognostic benefit for CT-based lung cancer screening in smokers12, the prognostic differences between the absence or presence of coronary calcification in smokers is of increasing clinical relevance. This question is particularly important as smokers with zero CAC may harbor non-calcified plaque which is not visualized on calcium scoring protocols13.
We sought to address these questions in the largest follow-up of CAC scanning yet undertaken.
Methods
The study cohort consisted of 44,042 consecutive asymptomatic individuals, free of known coronary heart disease (CHD), referred by their physicians for Electron Beam Tomography (EBT) in order to help refine individual CHD risk prediction. Patients were presumed to be free of clinical CHD based on a clinical history which was conducted by the referring physician. The dataset for this study represents the combination of data collected between 1991 and 2004 from three different centers in the US (Nashville TN, Columbus OH, and Torrance, CA). The combined population was predominantly of Caucasian ethnicity.
All screened individuals provided informed consent to undergo EBT and for the use of their blinded data for epidemiologic research. The general study received approval from the Human Investigations Committee, and separate Committee approval was obtained for patient interviews, collection of baseline and follow-up data, and corroboration of the occurrence of death.
Risk factor data collection
All study participants were given a questionnaire for the collection of demographic characteristics, as well as baseline cardiovascular risk factors. The following risk factors were considered in our study. 1) Cigarette smoking was considered present if a subject was an active smoker at the time of scanning. 2) Dyslipidemia was considered to be present for any individual reporting a history of high total cholesterol, high LDL-cholesterol, low HDL-cholesterol, high triglycerides, or current use of lipid-lowering therapy. 3) Diabetes was defined as baseline use of oral anti-diabetes medications or insulin. 4) Hypertension was defined as a self-reported history of high blood pressure or the use of antihypertensive medication. 5) Family history of premature CHD was determined by asking patients whether any member of their immediate family (parents or siblings) had a history of fatal or nonfatal myocardial infarction and/or coronary revascularization in a male relative <55 years or a female relatives <65 years in 36,010 (82% of the study population), whereas the age cutoff was <55 years of age in both male and female relatives in 8, 042 participants recruited from the Columbus center (18% of the study population)
EBT screening protocol
All subjects underwent EBT on either a C-100 or C-150 Ultrafast CT scanner (GE-Imatron, South San Francisco, California). With a tomographic slice thickness of 3 mm, a total of approximately 40 sections were obtained beginning at the level of the carina and proceeding caudally to the level of the diaphragm. Images were obtained with a 100-ms/slice scanning time, with image acquisition electrocardiographically triggered at 60% to 80% of the R-R interval. A calcified lesion was defined as ≥3 contiguous pixels with a peak attenuation of at least 130 Hounsfield units. Each lesion was then scored with the method developed by Agatston et al (Agatston Units [AUs]) 14.
Follow-up and mortality ascertainment
Patients were followed for a mean of 5.6 ± 2.6 years (range 1 to 13 years). Follow-up was completed in 100% of the patients. The primary end point for the study cohort was mortality from any cause. Ascertainment of mortality was conducted by individuals blinded to baseline historical data and EBT results. The occurrence of death was verified with the Social Security Death Index.
Statistical methods
The baseline characteristics of the study population are presented by smoking status. Age is presented as a continuous measure ± standard deviation (SD), and other risk variables are expressed as proportional frequencies. Age was compared across increasing CAC groups with analysis of variance techniques, and proportional frequencies of other risk variables were compared across increasing CAC groups with chi-square analysis. A p value <0.05 was considered statistically significant.
Annualized all-cause mortality rates were estimated by dividing the number of deaths by the number of person-years at risk. Mortality rates are first expressed for each CAC stratum group (0, 1-99, 100-399, and ≥400) and then stratified according to smoking status.
In addition, survival analysis was conducted with individual subject time to all-cause mortality data. Curves representing the cumulative probability of survival were generated with Kaplan-Meier estimates. Hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality were calculated for each CAC stratum with the Cox proportional hazards regression model, using CAC = 0 as the reference group. Two hierarchical models were constructed: Model 1: adjusted for age and sex; and Model 2: adjusted for age, sex, hypertension, dyslipidemia, diabetes mellitus, and family history of CHD. Schoenfeld residuals were calculated and visually interpreted to evaluate the validity of proportional hazards assumption. Interaction terms for gender and smoking in each CAC group were tested and discarded because of non-significance.
All statistical analyses were performed with STATA version 10 (STATA Corp., College Station, Texas).
Results
Clinical Characteristics of the Cohort
The final study population consisted of 44,042 asymptomatic individuals free of known cardiovascular disease prior to EBT. The average age was 54 ± 10 years. Approximately 14% of subjects were smokers (6,020 smokers and 38,022 non-smokers). Overall, smokers had a greater number of cardiac risk factors as compared to non-smokers (2.1±1.2 vs. 0.8±0.7, P<0.0001, Table 1).
Table 1. Clinical characteristics of subjects with and without a history of cigarette smoking.
| Nonsmokers (n = 38022, 86%) |
Smokers (n = 6,020, 14%) |
P Value | |
|---|---|---|---|
| Age (Years) | 55±11 | 53±10 | <0.0001 |
| Gender (female) (%) | 45% | 57% | <0.0001 |
| Hypertension (%) | 33% | 41% | <0.0001 |
| Diabetes (%) | 5% | 10% | <0.0001 |
| Dyslipidemia (%) | 26% | 52% | <0.0001 |
| Family history of CHD (%) | 33% | 62% | <0.0001 |
All-cause Mortality in Smokers and Non-smokers
There were 901 deaths (2.05%) in the study cohort overall. A total of 258 (4.29%) smokers died over the mean follow-up of 5.6 years, as opposed to 643 (1.69%) non-smokers (P<0.0001). The mean annualized mortality rate was 10.99 deaths per 1,000 person-years (95% confidence interval [CI]: 9.63-12.30) for smokers vs. 2.86 deaths per 1,000 person-years (95% CI 2.64 –3.09) among non-smokers. Furthermore, when the entire cohort was separated based on cardiac risk-factor subsets, smokers had consistently higher mortality rates than non-smokers (Table 2).
Table 2. All-Cause Mortality Rates by Cigarette Smoking status in the Overall Cohort.
| Nonsmokers | Smokers | |||
|---|---|---|---|---|
|
| ||||
| Rate per 1,000 person years at risk | 95% CI for rate | Rate per 1,000 person years at risk | 95% CI for rate | |
| Entire Cohort | 2.86 | 2.64-3.09 | 10.99 | 9.63-12.30 |
|
| ||||
| Age | ||||
|
| ||||
| <45 years | 0.83 | 0.60-1.16 | 5.41 | 3.66-8.02 |
| 45-64 years | 1.81 | 1.60-2.04 | 7.72 | 6.46-9.23 |
| ≥65 years | 9.14 | 8.22-10.16 | 33.16 | 27.54-39.94 |
|
| ||||
| Gender | ||||
|
| ||||
| Female | 2.11 | 1.84-2.42 | 9.02 | 7.53-10.81 |
| Male | 3.44 | 3.13-3.78 | 13.15 | 11.15-15.53 |
|
| ||||
| HTN | ||||
|
| ||||
| No | 2.19 | 1.99-2.42 | 8.21-9.83 | 6.85-9.83 |
| Yes | 6.29 | 5.53-7.16 | 15.04 | 12.73-17.75 |
|
| ||||
| Diabetes Mellitus | ||||
|
| ||||
| No | 2.42 | 2.22-2.63 | 9.93 | 8.67-11.35 |
| Yes | 14.78 | 12.34-17.69 | 20.37 | 15.16-27.38 |
|
| ||||
| Hyperlipidemia | ||||
|
| ||||
| No | 2.67 | 2.45-2.93 | 11.86 | 10.07-13.97 |
| Yes | 3.64 | 3.10-4.25 | 9.84 | 8.18-11.84 |
|
| ||||
| Family History of CHD | ||||
|
| ||||
| No | 2.63 | 2.39-2.89 | 12.45 | 10.39-14.93 |
| Yes | 3.53 | 3.08-4.05 | 9.84 | 8.33-11.61 |
In age-gender adjusted analysis (model 1), smoking was associated with at least a 4-fold increase of mortality (HR: 4.39, 95% CI: 3.78-5.10, p<0.0001). After adjusting for other relevant demographics and cardiac risk-factors (model 2), the HR for all-cause mortality among smokers was 3.73 (95% CI: 3.18-4.36). There were no deviations from the proportional hazards assumption in any multivariable model.
All-cause Mortality in Smokers and Non-smokers, as stratified by CAC
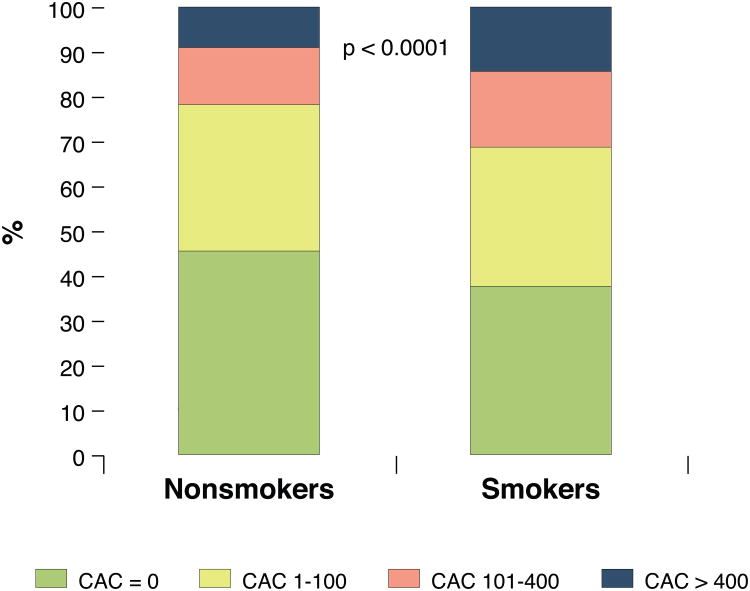
As shown in Figure 1 zero CAC was found in 38% of smokers as opposed to 46% of non-smokers (p<0.0001). CAC strata of 1-100, 101–400, and >400AUs were found in 31%, 17% and 14% of smokers. In comparison, the respective percentages were 32%, 12% and 9% for non-smokers.
Figure 1. CAC severity, based on smoking status.
Based on pre-specified CAC strata (1-100, 101-400, and >400 Agatston Units [AUs]), we found increased prevalence and severity of calcified subclinical atherosclerosis in smokers than in non-smokers. These CAC strata of 1-100, 101–400, and >400AUs were found in 31%, 17% and 14% of smokers, respectively. In comparison, the respective percentages were 32%, 12% and 9% for non-smokers.
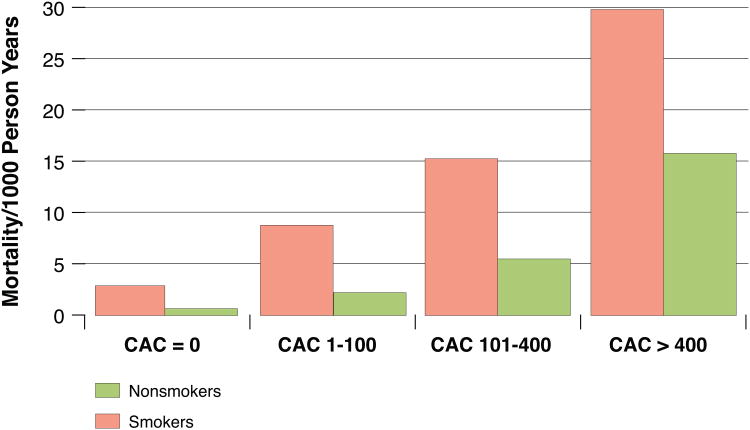
We found that all-cause mortality rates were higher in both smokers and non-smokers as baseline CAC score increased (Figure 2). The lowest mortality rate was observed in non-smokers without CAC (0.7 events per 1,000 person years), whereas smokers with CAC≥400 had the highest all-cause mortality rate (29.9 per 1,000 person years). Importantly, at each stratum of CAC score the mortality in smokers was noted to be higher than that of non-smokers in the CAC stratum above (for example, smokers in the CAC stratum of 1-100 had higher all-cause mortality rates than non-smokers in the CAC stratum of 101-400). Among smokers who died, CAC ≥400 was noted in 37% participants at baseline as compared to 13% of smokers who survived (p<0.0001).
Figure 2. Mortality rates (1000 person-years), according to smoking status and CAC.
The lowest mortality rate was observed in non-smokers without CAC (0.7 events per 1,000 person years), whereas smokers with CAC≥400 had the highest all-cause mortality rate (29.9 per 1,000 person years). Importantly, at each stratum of CAC score the mortality in smokers was noted to be higher than that of non-smokers in the CAC stratum above (for example, smokers in the CAC stratum of 1-100 had higher all-cause mortality rates than non-smokers in the CAC stratum of 101-400).
Using Cox proportional hazards regression, adjusting for demographics and CHD risk factors, we found that increasing CAC scores (1-99, 100-399, and ≥400) were associated with increased hazard of all-cause mortality among both smokers as well as non-smokers, compared to a CAC score of 0. However, the respective mortality hazard ratios as CAC group increased were 2.0 to 4.3-fold among smokers, and slightly higher at 2.6 to 8.0-fold among non-smokers (Table 3). In keeping with this, the smoking-CAC group interaction term was negative (HR of 0.75, p<0.001), suggesting that smoking exerts slightly less effect on mortality in the higher CAC groups than it does in those without CAC.
Table 3. All-Cause Mortality Hazard Ratios in Subjects with elevated baseline CAC compared to baseline CAC=0, stratified by Cigarette Smoking Status.
| Nonsmokers (n = 38,022, 86%) |
Smokers (n = 6,020, 14%) |
|
|---|---|---|
| Hazard ard Ratios (HRs) (95% CI) | Hazard Ratios (HRs) (95% CI) | |
| CAC 0 | Ref* | Ref† |
| CAC 1-99 vs. 0 | 2.62 (1.99-3.45)* | 2.04 (1.10-1.83)† |
| CAC 100-399 vs. 0 | 4.15 (3.11-5.54)* | 2.57 (1.62-4.05)† |
| CAC 400+ vs. 0 | 8.04 (6.09-10.61)* | 4.25 (2.72-6.63)† |
Adjusted for Age, gender, dyslipidemia, diabetes mellitus, HTN, family history of CHD
Comparing strata of positive CAC to CAC=0 in nonsmokers
Comparing strata of positive CAC to CAC=0 in smokers
We also compared the mortality, at each stratum of CAC elevation, between smokers and non-smokers (as opposed to calculating HRs for mortality as CAC increased within the smoking and non-smoking cohorts separately). At each stratum of baseline CAC elevation, smokers had higher mortality hazard ratios in multivariate analysis than non-smokers (Table 4). Thus, baseline CAC accurately stratified hazard ratios in both smokers and non-smokers, but at each stratum of CAC score that hazard was higher in the smoking group when directly compared to the non-smoking group. CAC also added significantly to the prediction of all-cause mortality among smokers, beyond traditional risk factors (chi square= 47.80, p<0.0001).
Table 4. All-Cause Mortality Hazard Ratios in Smokers as compared to Non-smokers, stratified by baseline CAC scores.
| Hazard Ratio for Smoking (95% CI) | |
|---|---|
| CAC 0 | 3.62 (2.28-5.75) |
| CAC 1-99 | 3.84 (2.82-5.22) |
| CAC 100-399 | 3.54 (2.57-4.89) |
| CAC 400 | 2.71 (2.12-3.48) |
Adjusted for Age, gender, dyslipidemia, diabetes mellitus, family history of CHD
We found that the percentage of female smokers with CAC >0 was 65%, as opposed to 57% of male smokers. However, the multivariate-controlled all-cause mortality hazard ratio for female smokers was 3.80 (95% CI, 2.96-4.87) and for male smokers was 3.66 (95% CI, 2.98-4.48), compared to their respective non-smoking counterparts. The smoking-gender interaction term was statistically non-significant (p=0.91) suggesting similar effect across gender.
Outcomes in smokers with zero CAC at baseline
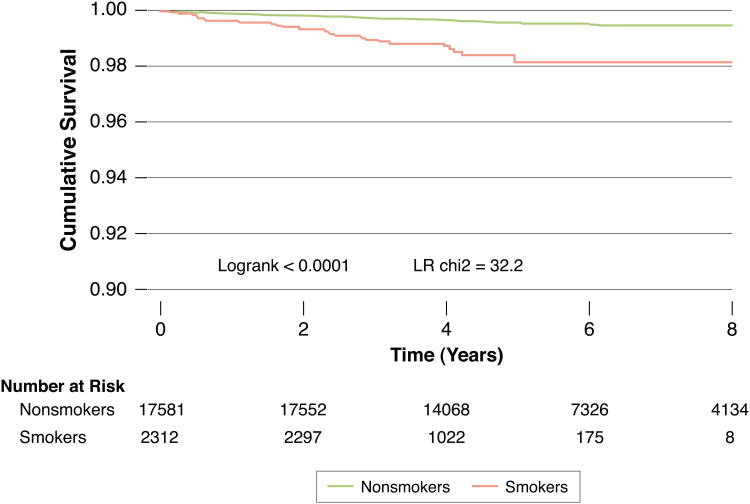
Smokers with zero CAC had an all-cause mortality rate of 3.31 deaths/1,000 person-years (95% CI: 2.31–4.74). This was in contrast to 0.67 deaths/1,000 person-years for non-smokers with CAC=0 (95% CI: 0.53–0.84). In the 19,898 individuals with CAC=0, mean 5.6 year all-cause survival was 99.6% for non smokers and 98.7% for smokers (p<0.001). Figure 3 demonstrates a Kaplan-Meier survival curve, based on Cox proportional hazards cumulative survival, for non-smoker and smokers among those with zero CAC (χ2 likelihood ratio= 32.2, p<0.0001). In our multivariable analysis, the HR for mortality in smokers without CAC was 3.62 (95% CI: 2.28-5.75) compared to non-smokers without CAC.
Figure 3. Survival in non-smokers and smokers with CAC=0.
This is a Kaplan-Meier survival curve among those with zero CAC, comparing non-smokers to smokers. In the 19,898 individuals with CAC=0, mean 5.6 year survival was 99.6% for non smokers and 98.7% for smokers (p<0.001). The HR for mortality in smokers without CAC was 3.62 (95% CI: 2.28-5.75) compared to non-smokers without CAC. Although the event rates are low and the absolute survival differences between smokers and non-smokers in the CAC=0 subgroup are small, our results demonstrate that the absence of CAC may not be as reassuring in those who smoke.
Discussion
In this combined cohort of 44,042 middle-age subjects, free of known CHD and followed for a mean of 5.6 years, we found that CAC is an independent predictor of all-cause mortality in both smokers and non-smokers. At each respective stratum of baseline CAC score, smokers were consistently found to have higher mortality rates than non-smokers in the CAC stratum above. Gender did not influence the mortality effect of smoking on CAC in our study. However, smokers with zero CAC were also shown to have higher relative mortality than non-smokers with zero CAC. These findings highlight the importance of smoking cessation even in those without measurable evidence of subclinical atherosclerotic disease.
Smoking and Atherosclerosis
Our findings are consistent with the known effect of smoking on mortality3, and add to the findings of prior research evaluating the interplay between smoking and atherosclerosis. Prior studies have associated pack-years of smoking exposure with the severity of angiographically determined atherosclerosis15. Cigarette smoking is a known independent predictor of new coronary atherosclerosis formation16. Similarly, smoking is a well documented risk factor for increased CAC, as assessed by cardiac CT9.
Clinical effects of smoking and CAC
The largest study to date analyzed a patient registry of 10,377 asymptomatic individuals undergoing EBT, with a follow-up of 5 years8. This cohort had a high prevalence of smoking (40%), considerably higher that our cohort and current national trends 1. Survival was 98.4% in non-smokers, compared with 96.9% in smokers. Multivariable relative risk ratios for mortality in smokers were elevated 1.8, 2.1, 3.5 and 4.5-fold higher, respectively, for patients with CAC scores of 11–100, 101–400, 401–1000, and >1000 (as compared with CAC scores of 0–10). However, this study did evaluate those without CAC8. In addition, we provide CAC-stratified mortality data for non-smokers as well as smokers-facilitating a direct comparison between these two the groups.
While we found that HRs for mortality did increase with each stratum of increasing baseline CAC in smokers, we also found that these increases were smaller in magnitude than the respective HRs for non-smokers in our cohort (Table 3). This finding is likely due to the increased all-cause mortality rates in the HR comparator groups of CAC=0 in smokers than in nonsmokers (3.3 Vs 0.7 deaths per 1,000 patient years, respectively [figure 2]). Also contributing to this was the finding of a negative interaction between smoking status and CAC group. In such, smoking has less effect on mortality as baseline CAC increased. We hypothesize that this is because much of the bad effects of smoking over a lifetime are “taken into account” by the high baseline CAC.
Importantly, baseline CAC severity does not fully explain the mortality risk of smoking. Indeed, when we controlled for baseline CAC in the multivariate analysis, we found that the HR for mortality remained significantly elevated at 3.31 (95% CI: 2.83-3.87, p<0.00001) for smokers compared to non-smokers. This finding should be interpreted in the context of our study endpoint of all-cause mortality, which incorporates non-cardiac causes of death. However, direct comparison of mortality HRs between smokers and nonsmokers found a similar magnitude of excess relative risk in smokers as each stratum of baseline CAC score increased (Table 4). This suggests that the relative effect of smoking was similar at each stratum of baseline CAC score.
Smoking in the Absence of Subclinical Atherosclerosis
While a CAC score of zero may be thought of as a “negative” cardiac risk factor 11, our results demonstrate that the absence of CAC may not be as reassuring in those who smoke. Although the event rates are low and the absolute survival differences between smokers and non-smokers in the CAC=0 subgroup are small (figure 3), the calculated hazard ratio of 3.62 for mortality is highly statistically significant and may translate into ongoing separation of the Kaplan-Meier survival curves over longer follow-up. Factors associated with an increased relative risk of mortality in smokers without CAC may be partly attributed to the potential presence of non-calcified plaque, which may be more prone to rupture than calcified plaque13.
Other explanations include the increased incidence of malignant arrhythmias and stroke in smokers, as well as death from other causes, in particular malignancies. Future study (which includes cause-of-death data) is needed to further elucidate the importance of cardiac events related to non-calcified plaque in smokers (as opposed to non-cardiac causes of death).
Clinical Implications
Our findings have a number of broad implications for risk prediction and preventive efforts in cardiology. Figure 2 demonstrates that the mortality rates in smokers at a given stratum of baseline CAC are higher than in nonsmokers in the next higher CAC stratum. As such, risk among smokers should be considered equivalent to those with higher subclinical atherosclerosis burden in the absence of smoking. Thus, we hope that the same sense of clinical foreboding extended by physicians to smokers with asbestos exposure also be extended to smokers found to have a moderate-large burden of CAC on testing.
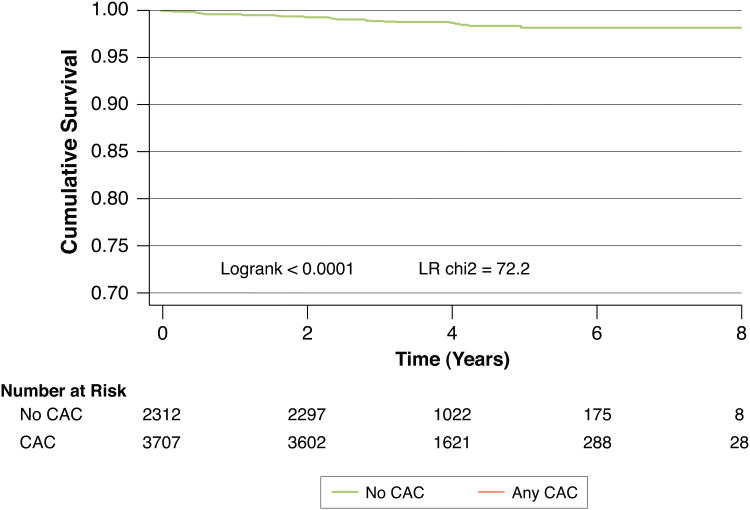
Our findings also extend to those without calcified subclinical atherosclerosis. Given the potential for increased future use of CAC quantification to assess individual cardiac risk17, as well as the expected increase in CT-based lung cancer screening in smokers12, it is important to evaluate whether all those with CAC=0 are uniformly at low risk for future events. While longer follow-up will be needed to confirm our finding of increased mortality in smokers with CAC=0 compared to nonsmokers with CAC=0, it seems prudent that the former group not be presently considered as low risk as the latter. Similarly, those smokers found to have any coronary calcification on chest CT imaging (for example, based on lung cancer CT screening) are at significantly increased future mortality risk compared to those without coronary calcification and should have their cardiac risk factors aggressively treated (Figure 4).
Figure 4. Survival in smokers without CAC Versus smokers with any CAC.
Trial evidence demonstrates a prognostic benefit for CT-based lung cancer screening in smokers and such screening may become much more widespread. Thus, the relative prognostic implications of visualizing any coronary calcification versus no coronary calcification on such screening are of clinical significance. Smokers with any CAC elevation are at significantly increased future mortality risk compared to those without CAC and would likely benefit from more aggressive cardiac risk factor modification.
We hope our findings may help animate smoking cessation discussions in those who have undergone CAC testing. However, whether CAC measurement can improve smoking cessation rates remains to be seen. The EISNER trial (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research)18 did not demonstrate improved smoking cessation in those randomized to undergo CAC testing. Unfortunately, the study was underpowered for this particular issue as the number of active smokers was small (less than 6% of the total study cohort).
Limitations
This is a retrospective analysis which cannot assess causality. All patients were referred for CAC screening and therefore do not represent a random sample of the population.
Another potential weakness is the self-reporting of risk factors. Data gathered by self report is limited by patient recall and thus subject to recall bias. While Hoff et al19 have shown a good reliability of self-reported histories of CHD risk factors in self-referred individuals for EBT scanning, potential “residual confounding” (which would possibly diminish the strength of association of risk factors with mortality) cannot be ruled out. Similarly, while the lack of a continuous risk variable might decrease the precision of point estimates of risk, the use of categorical risk factor data has been validated as an approach to clinical risk stratification20.
Unfortunately, we also do not have cardiac specific mortality available. However, the examination of death from all causes may allow for a more reliable prediction model without the possibility for cause of death misclassification21. We know from prior population-based studies that cardiac mortality accounts for about 35% of deaths in smokers and 27% of deaths in nonsmokers in our cohort age group3.
In addition, we do not have data regarding the number of pack years smoked and whether or not non-smokers were ex-smokers or never smoked in the past. Such data may have provided further insight into any dose-response relationship in this cohort. We plan to study this relationship further in the Multi-Ethnic Study of Atherosclerosis (MESA). It is of interest that several large epidemiologic studies have failed to find a significant dose-dependent correlation between cardiovascular risk and the pack-years of smoking exposure22-23.
Finally, we hypothesize that our results likely represents an underestimation of the effect of smoking. Population studies have shown that ex-smokers have higher cardiovascular event rates than never-smokers. Thus, the event rates reported in our non-smoking group may be higher than would be truly seen in those who never smoked. The event rates in our smoking group may also be an underestimation of the effect of continuous smoking exposure as we were unable to distinguish those who gave up smoking during our follow-up from those who continued to smoke. This potential underestimation of the effects of smoking in our analysis adds poignancy to the adverse findings we report above.
Conclusion
Smoking remains an important risk factor across the entire spectrum of subclinical coronary atherosclerosis, including amongst those with zero calcium scores. CAC remains an excellent way of risk stratifying both nonsmokers and smokers. Whether CAC quantification can motivate smoking cessation efforts deserves future study. Our data reinforce the notion that all smokers including those without subclinical coronary atherosclerosis, but especially those with increased CAC, should be strongly encouraged to quit.
Abbreviations
- CAC
Coronary Artery Calcium
- CT
Computed Tomography
- CHD
Coronary Heart Disease
- EBT
Electron Beam Tomography
- AU
Agatston Units
Footnotes
Financial disclosures and Conflicts of Interest: None to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.McElvaney NG. Smoking ban--made in Ireland, for home use and for export. N Engl J Med. 2004 May 27;350(22):2231–2233. doi: 10.1056/NEJMp048097. [DOI] [PubMed] [Google Scholar]
- 3.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004 Jun 26;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993 Nov;88(5 Pt 1):2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 5.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004 May 19;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Tracy RP, Psaty BM, Macy E, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997 Oct;17(10):2167–2176. doi: 10.1161/01.atv.17.10.2167. [DOI] [PubMed] [Google Scholar]
- 7.Howard G, Wagenknecht LE, Burke GL, et al. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998 Jan 14;279(2):119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 8.Shaw LJ, Raggi P, Callister TQ, Berman DS. Prognostic value of coronary artery calcium screening in asymptomatic smokers and non-smokers. Eur Heart J. 2006 Apr;27(8):968–975. doi: 10.1093/eurheartj/ehi750. [DOI] [PubMed] [Google Scholar]
- 9.McEvoy JW, Blaha MJ, Defilippis AP, et al. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010 Nov 9;56(20):1613–1622. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 10.McEvoy JW, Blaha MJ, Nasir K, Blumenthal RS, Jones SR. Potential use of coronary artery calcium progression to guide the management of patients at risk for coronary artery disease events. Curr Treat Options Cardiovasc Med. 2012 Feb;14(1):69–80. doi: 10.1007/s11936-011-0154-5. [DOI] [PubMed] [Google Scholar]
- 11.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009 Jun;2(6):692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011 Aug 4;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera JJ, Nasir K, Cox PR, et al. Association of traditional cardiovascular risk factors with coronary plaque sub-types assessed by 64-slice computed tomography angiography in a large cohort of asymptomatic subjects. Atherosclerosis. 2009 Oct;206(2):451–457. doi: 10.1016/j.atherosclerosis.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990 Mar 15;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Ramsdale DR, Faragher EB, Bray CL, Bennett DH, Ward C, Beton DC. Smoking and coronary artery disease assessed by routine coronary arteriography. Br Med J (Clin Res Ed) 1985 Jan 19;290(6463):197–200. doi: 10.1136/bmj.290.6463.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters D, Lesperance J, Gladstone P, et al. Effects of cigarette smoking on the angiographic evolution of coronary atherosclerosis. A Canadian Coronary Atherosclerosis Intervention Trial (CCAIT) Substudy. CCAIT Study Group. Circulation. 1996 Aug 15;94(4):614–621. doi: 10.1161/01.cir.94.4.614. [DOI] [PubMed] [Google Scholar]
- 17.Greenland P, Polonsky TS. Time for a policy change for coronary artery calcium testing in asymptomatic people? J Am Coll Cardiol. 2011 Oct 11;58(16):1702–1704. doi: 10.1016/j.jacc.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 18.Rozanski A, Gransar H, Shaw LJ, et al. Impact of Coronary Artery Calcium Scanning on Coronary Risk Factors and Downstream Testing The EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) Prospective Randomized Trial. J Am Coll Cardiol. 2011 Apr 12;57(15):1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoff JA, Daviglus ML, Chomka EV, Krainik AJ, Sevrukov A, Kondos GT. Conventional coronary artery disease risk factors and coronary artery calcium detected by electron beam tomography in 30,908 healthy individuals. Ann Epidemiol. 2003 Mar;13(3):163–169. doi: 10.1016/s1047-2797(02)00277-6. [DOI] [PubMed] [Google Scholar]
- 20.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 May 12;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 21.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999 Sep;34(3):618–620. doi: 10.1016/s0735-1097(99)00250-8. [DOI] [PubMed] [Google Scholar]
- 22.Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999 Mar;20(5):344–353. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- 23.Glantz SA, Parmley WW. Passive smoking and heart disease. Epidemiology, physiology, and biochemistry. Circulation. 1991 Jan;83(1):1–12. doi: 10.1161/01.cir.83.1.1. [DOI] [PubMed] [Google Scholar]