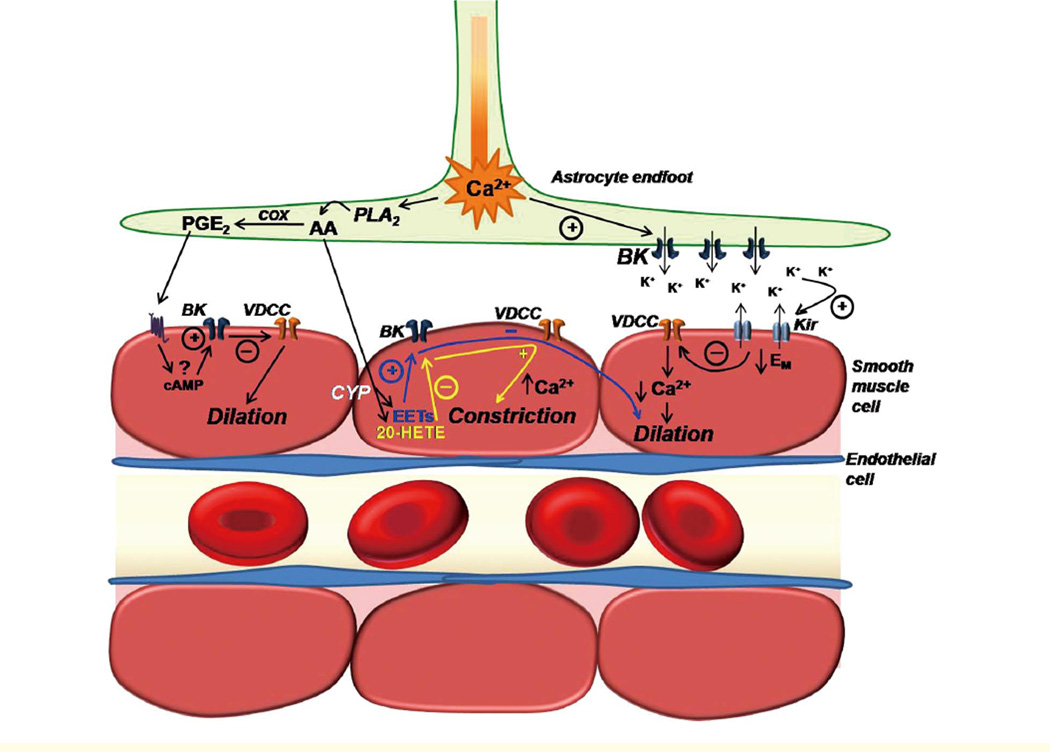
Figure 2.
Illustration depicting the involvement of parenchymal arteriolar smooth muscle cell (SMC) K+ channels in putative mechanisms of neurovascular coupling. Neuronal activity stimulates astrocytic metabotropic glutamate receptors (not shown) to produce a propagating rise in [Ca2+]i that terminates in perivascular endfeet. Increased astrocytic endfoot [Ca2+] activates BK channels to release K+ into the perivascular cleft. Moderate elevations in [K+]o in the perivascular cleft activate Kir channels in parenchymal arteriole SMCs, resulting in SMC membrane potential hyperpolarization, decreased Ca2+ entry through VDCCs, decreased [Ca2+]i, and vasodilation. Increased astrocytic endfoot [Ca2+] also activates the Ca2+ sensitive enzyme cytosolic phoshpolipase A2 (PLA2). PLA2 hydrolyzes membrane phospholipids to release the fatty acid arachidonic acid (AA). AA is then metabolized by cyclooxygenase (COX) and prostaglandin synthases to yield PGE2, or is believed to diffuse to the parenchymal arteriole SMC, where it is metabolized by cytochrome P-450 enzymes to generate EETs and/or 20-HETE. The mechanism of action of PGE2 on parenchymal arteriole SMCs is unknown, but based on studies done in pial arteries, it might involve cAMP-dependent activation of SMC BK channels, and subsequent SMC hyperpolarization and vasodilation. EETs formed in arteriolar SMCs by the metabolism of astrocytederived AA are proposed to activate SMC BK channels to elicit membrane hyperpolarization and vasodilation. Conversely, 20-HETE is believed to inhibit parenchymal arteriolar SMC BK channels resulting in membrane depolarization, activation of VDCCs, elevation of [Ca2+]i, and vasoconstriction.