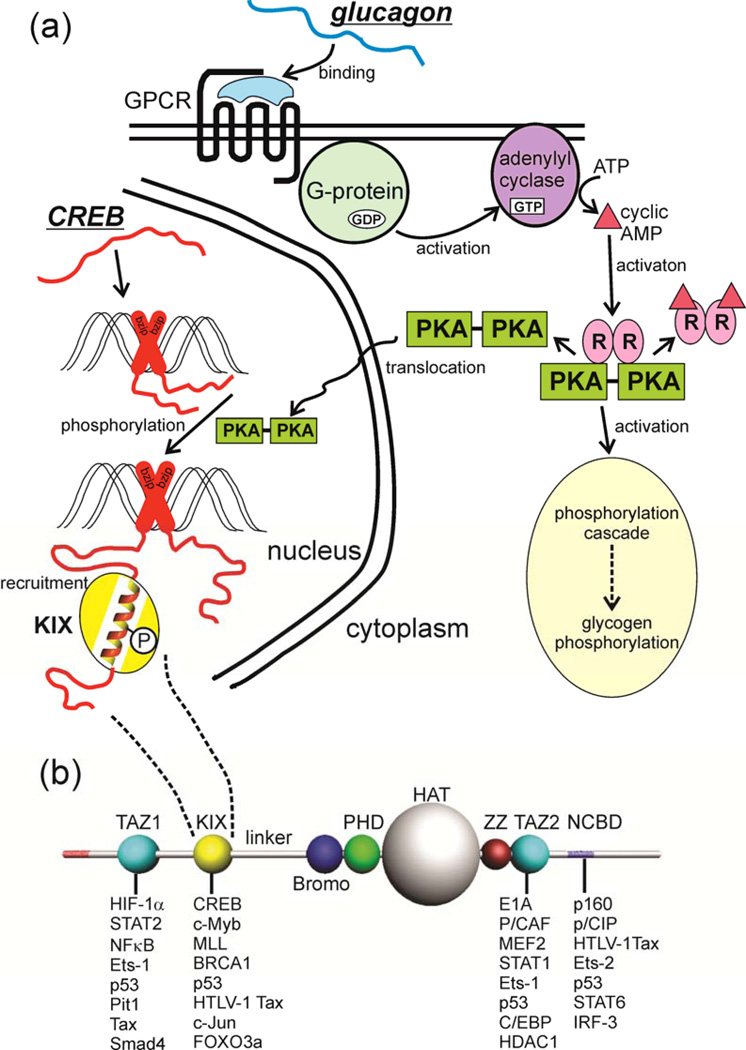
Figure 1. Intrinsic Disorder in Signaling.
(a) The metabolic hormone glucagon (an early example of intrinsic disorder in a functional molecule 144) binds to a structured, membrane-bound cell surface receptor, a G-protein-coupled receptor (GPCR) causing the translocation of the α subunit of the coupled G protein to the membrane-bound adenylyl cyclase, with concomitant formation of GTP from GDP. Cyclic AMP is generated, and activates protein kinase A (PKA), which has two downstream effects, firstly initiating the phosphorylation cascade that results in the phosphorylation of glycogen and the mobilization of stored glucose. The second effect is that activated PKA is translocated to the nucleus, where it phosphorylates the transcription factor cyclic-AMP response element binding protein (CREB), an intrinsically disordered protein. It appears that CREB is constitutively bound to the CRE DNA sequence through dimerization of the C-terminal basic leucine zipper domain (bzip, red cross). Phosphorylation of the kinase-inducible (KID) domain causes this domain to fold into a helical structure on the KIX domain of the transcriptional coactivator CREB-binding protein (CBP) (yellow), recruiting it to the promoter and promoting the transcription of downstream signal-response genes (reviewed in 145). In this case, intrinsically disordered proteins function both in the original reception of the signal and in the promotion of gene transcription in response to the signal. (b) Domain organization of CBP, showing a subset of the IDPs that bind to each of the four main interaction domains, the folded domains TAZ1, KIX and TAZ2, and the disordered (probably molten globular) NCBD.