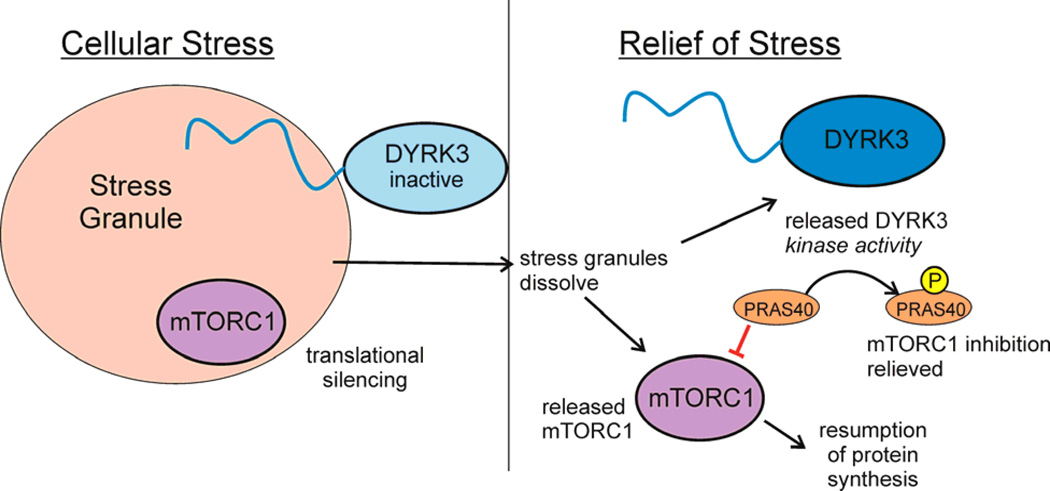
Figure 5. Disorder mediates stress-induced translational silencing.
Stress granules, which contain RNA and protein in a membraneless condensed particle, form and coalesce in response to cellular stress, sequestering proteins, including the dual-specificity kinase DYRK3 and mTORC1, the cellular factor that activates translation108. Translational silencing is mediated by DYRK3 under stress conditions in two ways, through stabilization of stress granules by the interaction of the disordered N-terminal tail, thus prolonging the sequestration of mTORC1. When cellular stress is relieved, DYRK3 and mTORC1 are released from the stress granules. Active DYRK3 acts as a kinase to phosphorylate PRAS40, relieving its inhibition of mTORC1 and allowing the resumption of protein synthesis. Figure adapted from 108 with permission.