Figure 2.
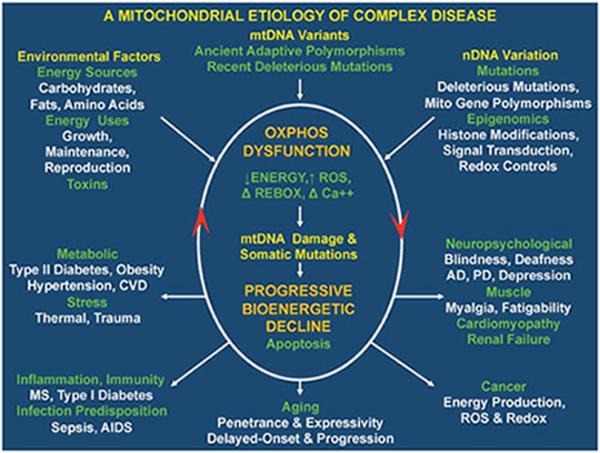
Integrated mitochondrial paradigm to explain the genetic and phenotypic complexities of the “complex” human diseases. An integrated model for the genetics and pathophysiology of complex diseases, aging, and cancer. The top of the figure indicates the three types of variation that impact individual mitochondrial oxidative phosphorylation (OXPHOS) robustness and hence risk for developing disease symptoms. These include nuclear DNA (nDNA) variation encompassing DNA sequence changes and epigenomic modification of gene regulation and signal transduction pathways, mitochondrial DNA (mtDNA) variation including recent deleterious mutations and ancient adaptive polymorphisms, and environmental influences encompassing the availability and demand for calories and inhibition of mitochondrial function by environmental insults. The central oval encompasses the pathophysiological basis of disease processes and the basis of disease progression. The primary defect is reduction in the energy transformation capacity of OXPHOS. This can result in reduced energy output, increased reactive oxygen species (ROS) production, altered redox status, and altered calcium homeostasis. The decline in OXPHOS efficiency can in turn perturb mitochondrial biogenesis, increase ROS production, impair mitophagy, and so on, resulting in the progressive increase in mtDNA damage and somatic mutations and further decline in mitochondrial function. Once mitochondrial function falls below the bioenergetic threshold of a tissue, symptoms ensue. Continued energetic failure can initiate cell destruction by apoptosis or necrosis. The lower five derived disease categories summarize the phenotypic outcomes of perturbed mitochondrial energy transformation. The bottom arrow shows the effect of the stochastic accumulation of somatic mtDNA mutations, resulting in delay-onset and progressive course of diseases and aging. The right arrow indicates clinical problems that can result from reduced energy production in the most energetic tissues, the brain, heart, muscle, and kidney. The number and severity of symptoms in these organs reflect the degree and specific nature of the mitochondrial defect. The arrow to the left indicates the metabolic effects of mitochondrial dysfunction that result in the perturbation of the body's energy balance. This results in the symptoms of the metabolic syndrome. The arrow toward the lower right corner indicates mitochondrial alterations that are critical for cancer initiation, promotion, and metastasis. The arrow to the lower left corner outlines the hypothesized inflammatory and autoimmune responses that may result from the chronic introduced into the bloodstream of the mitochondria's bacteria-like DNA and N-formyl methionine proteins.
