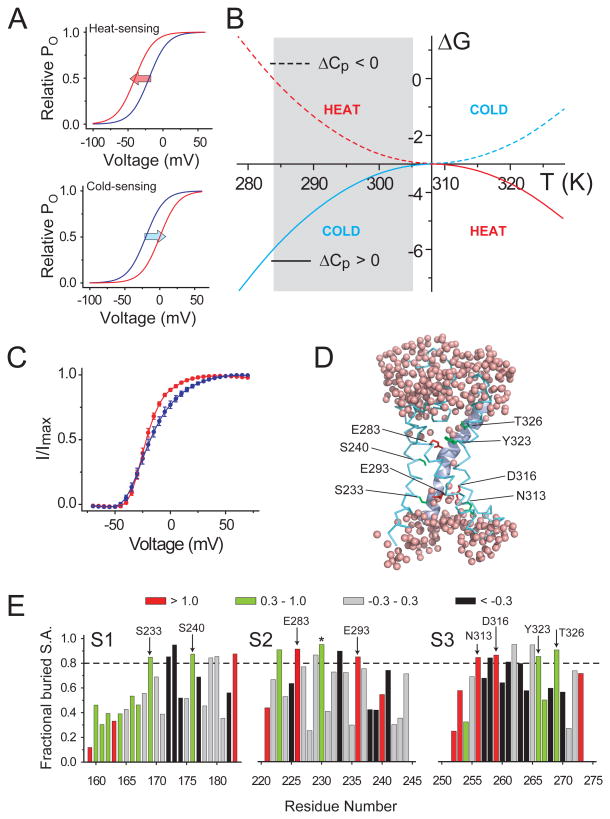
Figure 1. Design template for engineering a temperature modulated ion channel.
(A) Arbitrary relative open probability vs voltage (POV) curves for a heat-sensing channel (top) and a cold-sensing channel (bottom) at two temperatures (red: high temperature, blue: low temperature). Arrows indicate the shifts in the curve on heating. (B) ΔG vs T profiles simulated using the equation: ΔG(T) = ΔHC + ΔCP(T−TC) − TΔCPln(T/TC) for two processes, one with a positive ΔCP (solid curve, ΔCP = 3 kcal/(mol.K)) and the other with a negative ΔCP (dotted curve, ΔCP = −3 kcal/(mol.K)) (see also Fig. S1). In both cases. the critical temperature, TC, was 308K (35°C), at which the change in enthalpy, ΔHC, was −3 kcal/mol. The heat sensing and cold sensing regimes of the curves are indicated by the red and blue colors respectively. (C) Relative POV curve of the wild-type Shaker KV channel at 28°C (red) and 8°C (blue) (measured from tail currents) (see also Fig. S1). (D) A model of a hydrated voltage-sensor, deduced from the crystal structure of the KV1.2/2.1 paddle chimera (see Extended experimental procedures in Supplementary Material), showing occupancy of water within the crevices of the voltage-sensor and the sites that were perturbed in this study. The S4 helix is shown as a cartoon while the S1-S3 helices are shown as ribbons. (E) Fractional buried surface area of different residues in the transmembrane segments S1-S3 (residues numbered according the 2R9R structure). The dotted horizontal lines indicate the cut-off for buried surface areas. The bars are colored according to the polarity index of the residue position, deduced from an alignment of 360 KV channel sequences. Residues targeted in this study are marked with arrows, with the residue numbers corresponding to Shaker KV channel. The residue marked with an asterisk, while enriched in polar residues in the alignment, is a hydrophobic residue in Shaker KV channel and was not studied here.