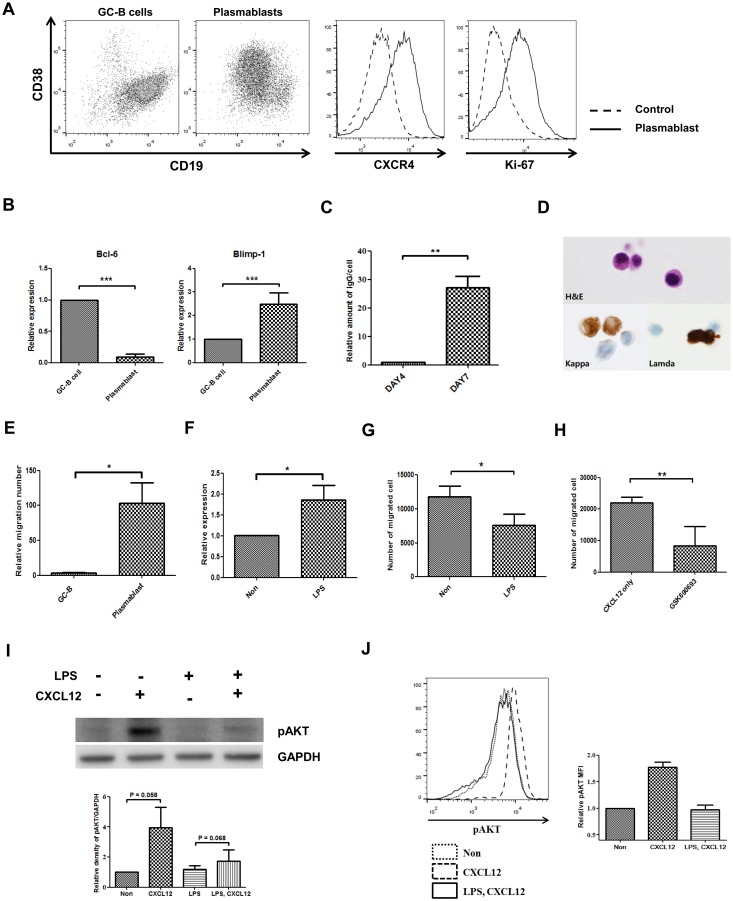
Fig 4. LPS reduces CXCL12-mediated migration via RGS1-AKT in plasmablasts.
GC-B cells were cultured in combination with IL-2, IL-4, and IL-21 for 7 days and differentiated into plasmablasts. The cultured plasmablasts were stimulated with 10 ng/mL LPS for 18 hours. RGS1 expression and AKT phosphorylation were assessed in LPS-treated plasmablasts, in addition to assessment using the transwell migration assay. A) CXCR4, CD38, CD19, and Ki-67 expression levels in the cultured plasmablasts. B) Expressions of Bcl-6 and Blimp-1 were measured by real-time PCR at GC-B cells and cultured plasmablast. C) Amount of secreted immunoglobulin was measured at culture day4 and culture day 7. D) After 7 days of culture, purified GC-B cells differentiated to plasmablasts. The cytoplasm is abundant and the nucleus characteristically shows the eccentric position. The nucleus shows fine reticular or mildly blocked chromatin pattern. These plasmablasts show strong cytoplasmic staining for the kappa or the lambda light chain immunocytochemical staining. (H&E, Hematoxylin and eosin stain × 1,000; Kappa, anti-kappa light chain, × 1,000; Lamda, anti-lambda light chain, × 1,000). E) Plasmablasts demonstrate high migratory properties towards CXCL12. F) LPS treatment induces RGS1. G) LPS reduces CXCL12-mediated transwell migration. H) AKT-specific inhibitors reduce migration. I) LPS reduces CXCL12-mediated AKT phosphorylation. Representative western blot of four repetitive experiments is shown. Quantification of phospho-AKT expression was shown in Graph. Phospho-AKT expression was normalized to control levels and corrected for loading differences using GAPDH. J) Intracellular staining of phospho-AKT showed the reduction of the phospho-AKT by LPS treatment. Representative data of two repetitive experiments was shown. Average mean fluorescence intensity (MFI) was shown in graph (*p <0.05; **p <0.005***p <0.0005).