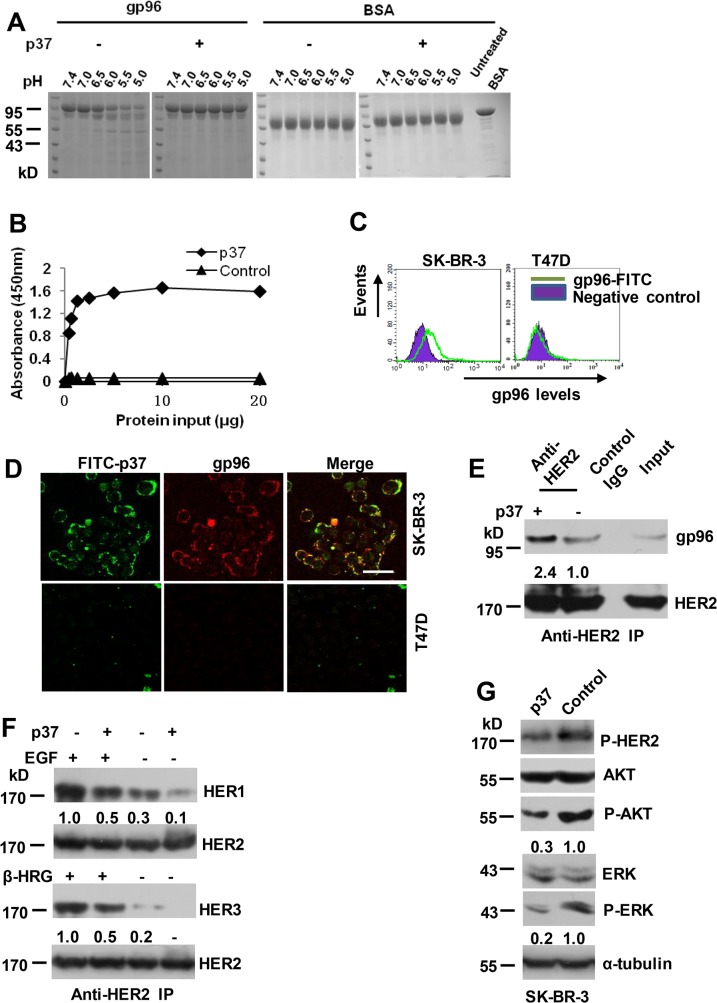
Fig 1. gp96 conformational changes are required for facilitating HER2 dimerization.
(A) 10 μM of purified gp96 was treated with 100 μM of p37 (444–480 aa) or control (61–100 aa) peptides and subjected to trypsin digestion at the indicated pH values. BSA served as a control. (B) ELISA analysis of interaction between gp96 and p37 or control peptides. (C) Flow cytometric analysis of cell surface levels of gp96. (D) SK-BR-3 and T47D cells were cultured in presence of FITC-labeled p37 for 30 minutes, and then stained by immunofluorescence (TRITC) using an anti-gp96 antibody. Scale bar, 40 μm. (E-F) SK-BR-3 cells were treated with 20 μg/ml of p37 or control peptide for 30 min. Cell lysates were immunoprecipitated with anti-HER2 antibody (E). SK-BR-3 cells were pre-treated with EGF (50 ng/ml) or β-heregulin (100 ng/ml) for 15 min, and then cells were treated and analyzed as in E (F). Numbers below the blot indicates quantification shown on Western blot after normalization to HER2. (G) Western blot assay of cell lysates of SK-BR-3 cells treated with 20 μg/ml of p37 or control peptide for 8 h. The ratios of P-AKT to AKT and P-ERK to ERK were calculated, and the values were shown. The ratios in control peptide-treated cells were arbitrarily taken as 1.0.