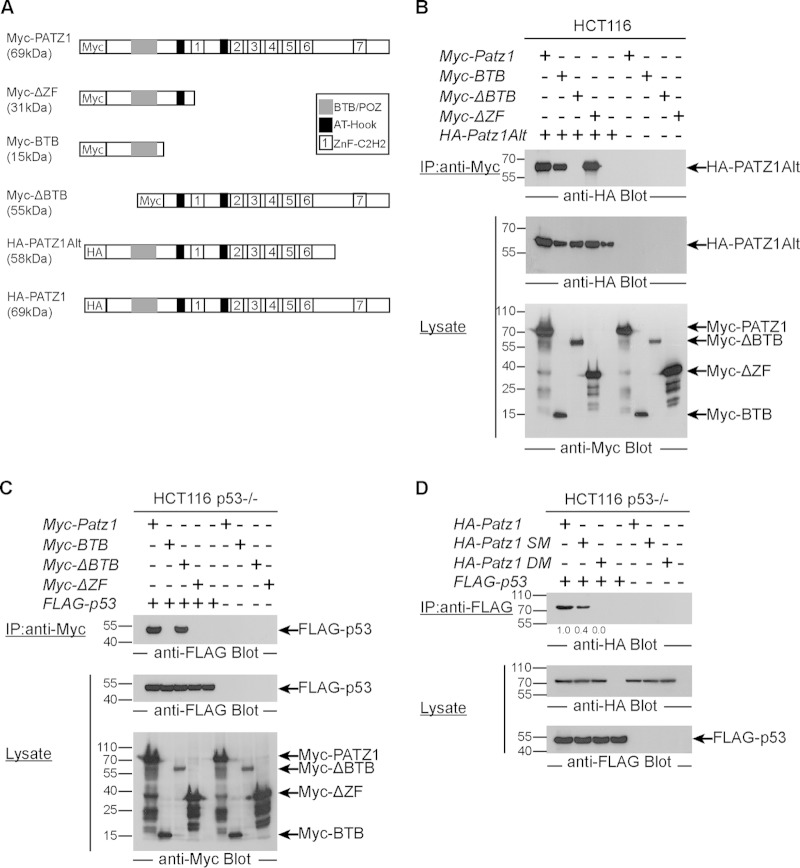
FIG 4.
The C-terminal tail of PATZ1 is required for binding p53. (A) Schematic representation of PATZ1 truncations. N-terminal epitope tags are indicated by boxes with HA or Myc. The names and expected molecular masses of the PATZ1 truncations are indicated on the left. (B) BTB domains are necessary for PATZ1 and PATZ1Alt heterodimerization. (Top) Anti-Myc immunoprecipitation followed by anti-HA antibody blotting reveals the presence of a complex of Myc-PATZ1 and HA-PATZ1Alt only in cells expressing BTB containing Myc-PATZ1 proteins (lanes 1, 2, and 4). Myc-ΔBTB cannot bind to HA-PATZ1Alt (lane 3). (Middle and bottom) Lysates of transfected HCT116 cells blotted with anti-HA and anti-Myc show that PATZ1, truncation variants of PATZ1, and PATZ1Alt proteins are expressed at equal levels. (C) The C-terminal ZFs of PATZ1 are necessary for p53 binding. (Top) Anti-Myc immunoprecipitation followed by anti-FLAG antibody blotting reveals the presence of FLAG-p53 in complex with Myc-PATZ1 (lane 1) and Myc-ΔBTB (lane 3) but not Myc-BTB (lane 2) or Myc-ΔZF (lane 4) in HCT116 p53−/− cells transfected with the indicated plasmids. Binding is specific, as no coimmunoprecipitation is evident in lysates lacking FLAG-p53 (lanes 6 to 9). (Middle and bottom) Lysates of transfected cells show that FLAG-p53 (middle) and Myc-PATZ1 and its truncations (bottom) are expressed at equal levels in transfected cells. Some Myc epitope-tagged PATZ1 truncations revealed lower-molecular-weight degradation products, but the proteins of interest at the expected size are indicated by arrows on the right. (D) Negatively charged residues in the ZF domain are required for PATZ1-p53 binding. (Top) Anti-FLAG immunoprecipitation followed by anti-HA antibody blotting reveals the presence of HA-PATZ1 (lane 1) in complex with FLAG-p53 in HCT116 p53−/− cells transfected with the indicated plasmids (top). PATZ1 with a single mutation (HA-PATZ1 SM) has attenuated binding to p53 (lane 2), while PATZ1 with a double amino acid mutation (HA-PATZ1 DM) cannot bind p53 (lane 3). Binding is specific, as no coimmunoprecipitation is evident in lysates lacking FLAG-p53 (lanes 5 to 8). (Middle and bottom) Lysates of transfected cells show that HA-PATZ1 and its mutants (middle) and FLAG-p53 (bottom) are expressed at equal levels in transfected cells.