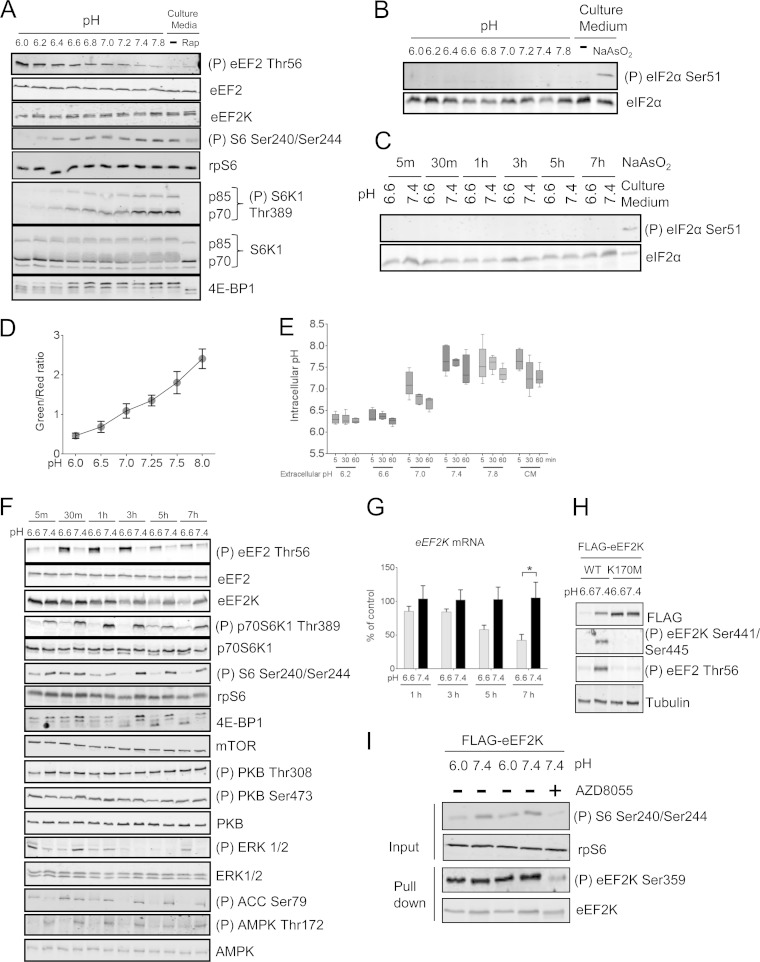
FIG 1.
Extracellular acidosis inhibits mTORC1 and activates eEF2K. (A) HEK293 cells were incubated in pH-buffered medium or normal culture medium for 30 min with/without 100 nM rapamycin (Rap). (B and C) HEK293 cells were incubated in pH media buffered to different pHs for the indicated periods of time or in culture medium in the presence or absence of 0.5 mM NaAsO2 for 2 h. The levels of total and phospho-eIF2α (Ser51) were analyzed by Western blotting. (D) HEK293 cells transfected with mCherry/de4GFP were under high-[K+]/nigericin pH clamp from pH 6.0 to 8.0 for 10 min. An increase in red fluorescence indicates an acidic pH shift. The standard curve was established by determining the relationship between pH and the green/red fluorescence ratio. (E) HEK293 cells transfected with mCherry/de4GFP were cultured in either pH-buffered or culture medium (CM) for the indicated times. The intracellular pH of cells was determined via interpolation of green/red fluorescence ratio into the standard curve illustrated in panel D. (F) HEK293 cells were incubated in medium buffered at pH 6.6 or 7.4 for the indicated times. (G) HEK293 cells were cultured at pH 6.6 or 7.4 buffered medium for up to 7 h, and expression of EEF2K mRNA was quantified by real-time RT-PCR. Results are given as means ± standard errors (SE) from 3 independent experiments and expressed as a percentage of the control (pH 7.4). *, 0.01 ≤ P < 0.05 as determined by two-way analysis of variance (ANOVA). (H) HEK293 cells were transfected with vectors for wild-type or K170M (kinase-dead) FLAG-tagged eEF2K; 24 h later, cells were cultured at pH 6.6 or 7.4 for 7 h before lysis. (I) HEK293 cells were cultured at pH 6.0 or 7.4 buffered medium for 30 min, eEF2K was immunoprecipitated from the lysates, and Ser359 phosphorylation was analyzed by Western blotting. For all SDS-PAGE and Western blot experiments, data shown are representative of three independent experiments.