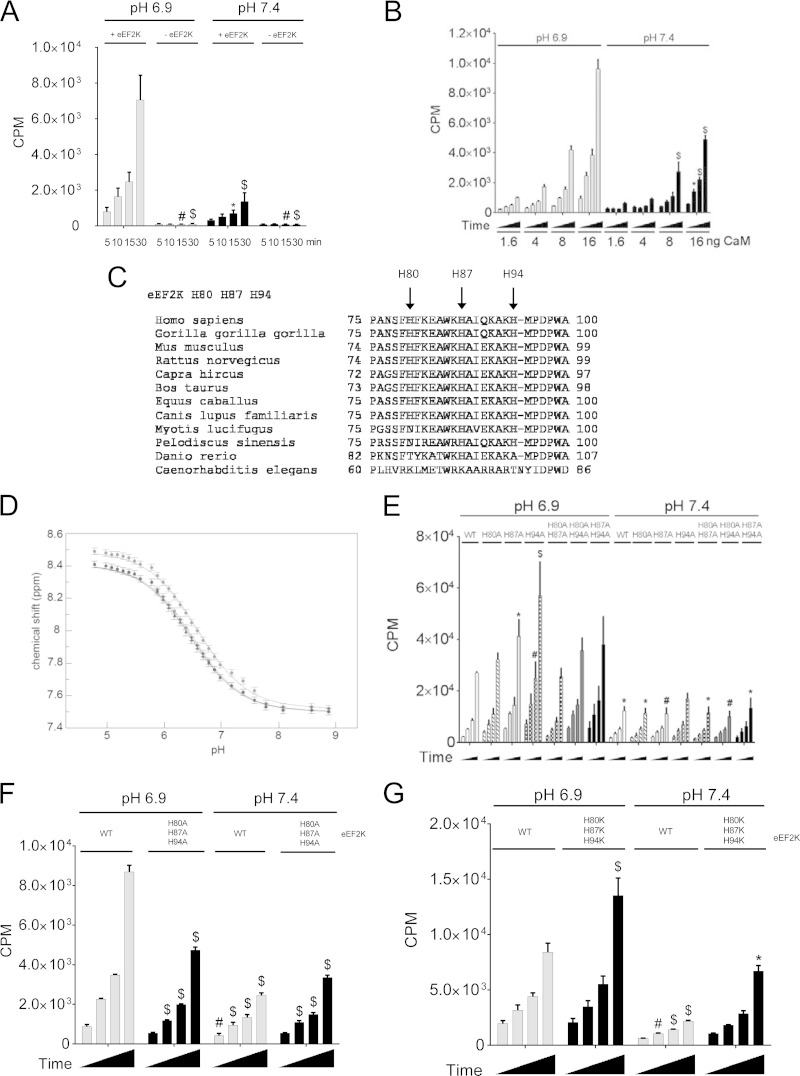
FIG 5.
Histidine residues within the CaM-binding site of eEF2K are important for eEF2K activation under low pH. (A) eEF2K activity assay with or without wild-type recombinant eEF2K at either pH 6.9 or 7.4. (B) eEF2K activity assays with the indicated amount of CaM per assay. (C) Sequence alignment of eEF2K CaM-binding domain among species. (D) Chemical shift titration curves for the CH2 protons of the imidazole side chains of the 3 histidine residues in eEF2K(82–100). (E) Activity of WT eEF2K compared to the single or double H80A/H87A/H94A mutants. (F) Comparison of the activity of wild-type eEF2K with that of the H80A/H87A/H94A triple mutant. (G) eEF2K assay for eEF2K(H80K/H87K/H94K). Results are means ± SE, n = 3. P values were obtained by two-way ANOVA followed by Dunnett's test. *, 0.01 ≤ P < 0.05; #, 0.01 < P ≤ 0.001; $, P < 0.001.