LETTER
Two-pore channels (TPCs) are ancient ion channels that reside within acidic organelles, such as endosomes and lysosomes (1). TPCs are the proposed target of the second messenger nicotinic acid adenine dinucleotide phosphate (NAADP), which mobilizes Ca2+ from “acidic Ca2+ stores” (2). However, fundamental aspects of TPC biology—how they activate and their native ionic permeability (3–8)—remain controversial (reviewed in references 9 and 10).
Photolabeling studies using a radioactive NAADP-derived probe (5N3-[32P]NAADP) have suggested that NAADP may not directly bind to TPCs but may stimulate channel activity via small NAADP-binding proteins (∼23 kDa) associated with the TPC complex (11–14). One piece of evidence supporting this idea is the preservation of photolabeling in TPC knockout mice (11). For example, as shown in Fig. 1A, NAADP-specific labeling at 23 kDa is apparent in the knockout TPC1−/− mouse sample and selectively displaced by unlabeled NAADP, with affinity similar to that of wild-type mice. These data indicate that TPCs are unlikely the direct targets of NAADP.
FIG 1.
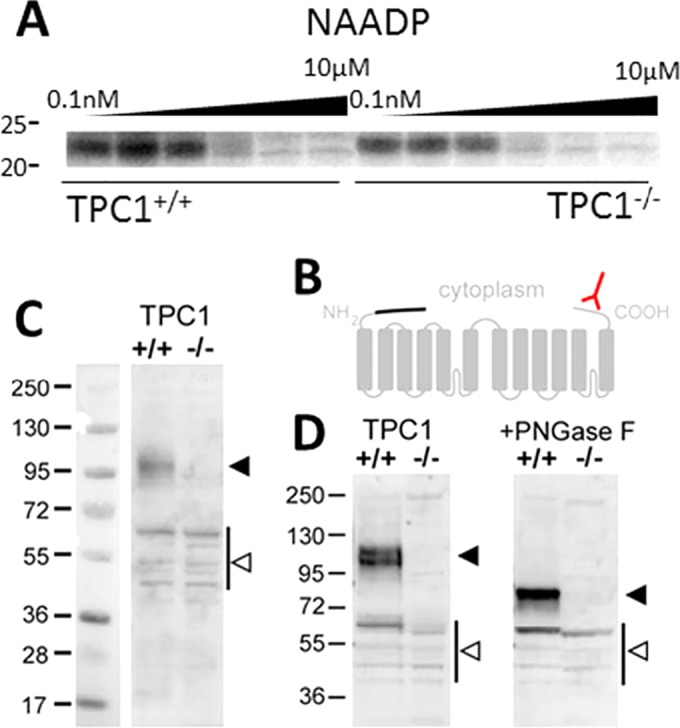
TPC1 protein is absent in transgenic TPC1 mice. (A) Photolabeling data using 5N3-[32P]NAADP showing specific photolabeling of an ∼23-kDa candidate NAADP binding protein in mouse pancreatic samples from a matched littermate mouse (TPC1+/+ [left]) and a TPC1 knockout mouse (TPC−/− [right]; Tpcn1LEXKO-471). Further detail is available in reference 11. (B) Schematic of TPC1 membrane topology to highlight the COOH-terminal epitope (red) present in wild-type TPC1 and the putative NH2-terminal truncation of TPC1B (the missing sequence is shown with a black line). (C and D) Western blot analysis of liver samples from wild-type (+/+) and Tpcn1LEXKO-471 (–/–) mice described in reference 11 using an antibody raised to the C terminus of TPC1 (ab80961; Abcam). (D) Samples were treated with peptide-N-glycosidase F (PNGase F) (right) to remove N-linked oligosaccharides as described in reference 17. Note the absence of major immunoreactive bands (solid arrowheads) in the transgenic mice. Bands present in both samples (open arrowheads) are likely nonspecific, but we cannot rule out the possibility of severely truncated, nonfunctional TPC1 variants that escape inactivation. Numbers at the left of the gels are molecular masses (in kilodaltons).
In a recently published study (15), Ruas and colleagues challenge this interpretation. Their data suggest the existence of a mouse TPC1 variant (TPC1B) with a truncated NH2-terminal domain compared to the previously studied TPC1 isoform (TPC1A). If this TPC1B variant were to be prevalently expressed, or if gene trap skipping occurred in the previously used Tpcn1 null line (Tpcn1LEXKO-471) to preserve TPC1A expression, then for either of these reasons, samples would not be TPC1 null. This would invalidate the conclusion of prior photolabeling experiments (11). We concur that it is important to investigate TPC1 expression to confirm the status of this mouse line as TPC1 null.
In this regard, TPC1B represents an NH2-terminal truncation of 69 residues relative to TPC1A (Fig. 1B). While no evidence demonstrating endogenous expression of TPC1B protein or the abundance of the TPC1B variant relative to wild-type TPC1A was provided, we note that a similarly truncated construct (85 residues) has previously been shown by us to be retained in the endoplasmic reticulum (ER) (16). Indeed, ER retention of TPC1B is increased in colocalization data presented by Ruas and colleagues (see Fig. 4D in reference 15). Further, the blot of overexpressed TPC1B (Fig. 4B in reference 15) shows evidence of impaired glycosylation (important for TPC function [17]), suggesting that TPC1B may be poorly expressed.
To assess TPC1 expression in the Tpcn1LEXKO-471 mouse model used previously in our photolabeling experiments (11), Western blotting was performed with a validated antibody (16, 18) capable of recognizing a COOH-terminal epitope present in both TPC1 isoforms (Fig. 1B). The proposed TPC1B truncation would be similar in size to full-length TPC1A (predicted at 87 versus 94 kDa for nascent forms). Our data revealed a complete loss of TPC1 immunoreactivity at this size (Fig. 1C and D). This result underscores prior conclusions (11–13). Repetition of our published photolabeling experiments with Tpcn1T159 samples characterized by Ruas and colleagues (15) is expected to yield a similar result. Comparison of whole animal phenotypes between different TPC1−/− strains will require further investigation.
In conclusion, the Tpcn1LEXKO-471 mouse line can be employed by the community as a Tpcn1−/− sample.
Footnotes
For the author reply, see doi:10.1128/MCB.00083-15.
REFERENCES
- 1.Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S. 2014. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci Signal 7:ra109. doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel S, Docampo R. 2010. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol 20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans MA, Galione A, Zhu MX. 2009. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. 2009. Essential requirement for two pore channel 1 in NAADP-mediated calcium signalling. J Cell Biol 186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. 2009. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch 458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. 2012. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jha A, Ahuja M, Patel S, Brailoiu E, Muallem S. 2014. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J 33:501–511. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin-Moshier Y, Keebler MV, Hooper R, Boulware MJ, Liu X, Churamani D, Abood ME, Walseth TF, Brailoiu E, Patel S, Marchant JS. 2014. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc Natl Acad Sci U S A 111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchant JS, Patel S. 2013. Questioning regulation of two-pore channels by NAADP. Messenger 2:113–119. doi: 10.1166/msr.2013.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan AJ, Galione A. February 2013. Two-pore channels (TPCs): current controversies. Bioessays doi: 10.1002/bies.201300118. [DOI] [PubMed] [Google Scholar]
- 11.Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. 2012. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J Biol Chem 287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT. 2012. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J Biol Chem 287:2308–2315. doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walseth TF, Lin-Moshier Y, Weber K, Marchant JS, Slama JT, Guse AH. 2012. Nicotinic acid adenine dinucleotide 2′-phosphate binding proteins in T lymphocytes. Messenger 1:86–94. doi: 10.1166/msr.2012.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchant JS, Lin-Moshier Y, Walseth T, Patel S. 2012. The molecular basis for Ca2+ signalling by NAADP: two-pore channels in a complex? Messenger 1:63–76. doi: 10.1166/msr.2012.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruas M, Chuang KT, Davis LC, Al-Douri A, Tynan PW, Tunn R, Teboul L, Galione A, Parrington J. 2014. TPC1 has two variant isoforms, and their removal has different effects on endo-lysosomal functions compared to loss of TPC2. Mol Cell Biol 34:3981–3992. doi: 10.1128/MCB.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churamani D, Hooper R, Rahman T, Brailoiu E, Patel S. 2013. The N-terminal region of two-pore channel 1 regulates trafficking and activation by NAADP. Biochem J 453:147–151. doi: 10.1042/BJ20130474. [DOI] [PubMed] [Google Scholar]
- 17.Hooper R, Churamani D, Brailoiu E, Taylor CW, Patel S. 2011. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J Biol Chem 286:9141–9149. doi: 10.1074/jbc.M110.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hockey L, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S. 2015. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci 128:232–238. doi: 10.1242/jcs.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]


