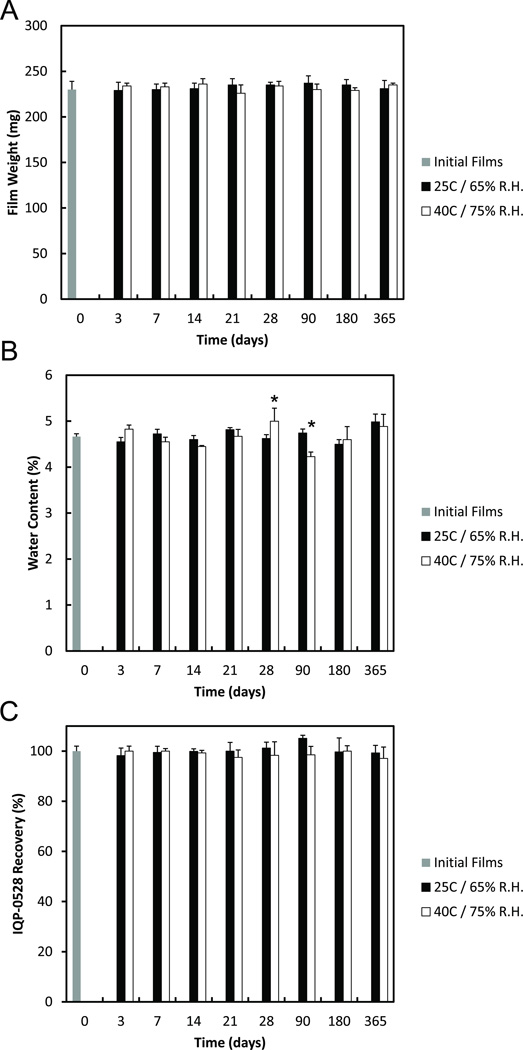
Figure 3.
(A) The stability of IQP-0528 film weight over 12 months. After manufacturing, the weight of the films was measured to provide a baseline film weight (grey). The films were then packaged into individual air-tight light-proof pouches and stored under 25°C / 65% R.H. (black) and 40°C / 75% R.H. (white), respectively. (B) The stability of IQP-0528 film moisture content (% water) over 12 months. After manufacturing, the moisture content of the films was measured to provide a baseline film weight (grey). The films were then packaged into individual air-tight light-proof pouches and stored under 25°C / 65% R.H. (black) and 40°C / 75% R.H. (white), respectively. (C) The stability of IQP-0528 from the film over 12 months. After manufacturing, the IQP-0528 content of the film was measured via HPLC as a percent recovery of the loaded amount (grey). The films were then packaged into individual air-tight light-proof pouches and stored under 25°C / 65% R.H. (black) and 40°C / 75% R.H. (white), respectively. For each evaluation, at the specified time points, the films were removed from storage and allowed to equilibrate to ambient temperature. The films were then removed from their packaging and evaluated for their stability. ± standard deviation; n = 3; * p < 0.05