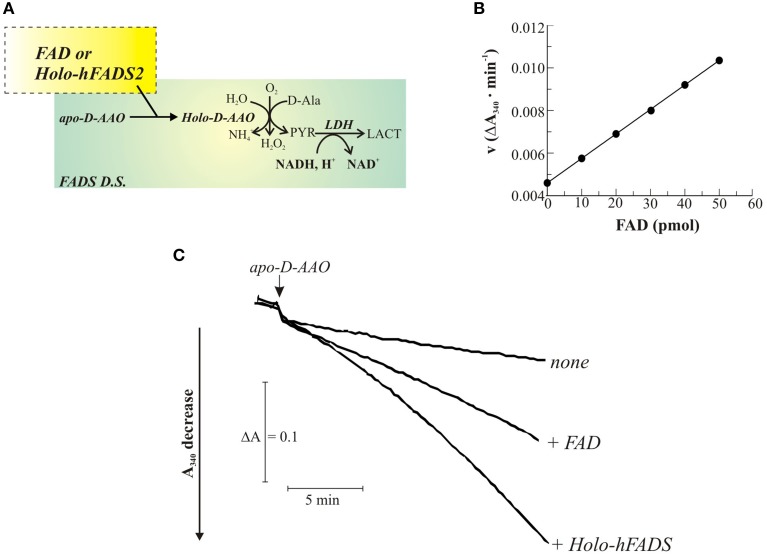
Figure 4.
FAD delivery from 6His-hFADS2 to the client apo-D amino acid oxidase. The release of FAD from the purified recombinant 6His-hFADS2 to apo-DAAO was assayed enzymatically, as described in Materials and Methods and schematized in (A), by measuring the activity of reconstituted holo-DAAO (derived from FAD binding to apo-DAAO). (B) Calibration curve obtained with a FAD standard. In (C) typical traces are shown. Apo-DAAO was added in the absence (none) or in the presence of purified 6His-hFADS2 (Holo-hFADS2, 1.2 μg, 20 pmol) or in the presence of commercial FAD (20 pmol) at 37°C in 100 μL of 50 mM Tris-HCl, pH 7.5. Reconstituted holo-DAAO activity was measured as described in Materials and Methods.