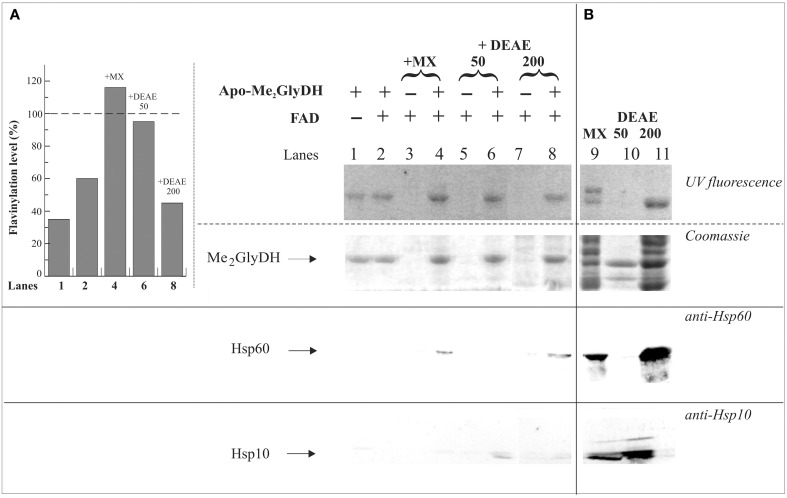
Figure 7.
Evidences of Me2GlyDH flavinylation and identification of possible interactors. In (A) 6His-apo-Me2GlyDH (10 μg) was incubated at 37°C in flavinylation medium made of 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2 5 mM, 0.5% Triton X-100, 5 mM ATP, in the absence or presence of 20 μM FAD. Where indicated rat liver mitochondrial matrix (MX) (lane 4) or MX fractions eluted onto a DEAE-Sephacel column with 50 and 200 mM NaCl (D50 and D200 fractions, 100 μg each, lanes 6 and 8, respectively) were added to the reaction mixture. As a control the same matrix and DEAE fractions were incubated in the same experimental condition in the absence of apo-Me2GlyDH and in the presence of 20 μM FAD (lanes 3, 5, 7). After 1 h incubation, each sample was passed on a Ni-Chelating Sepharose to re-isolate the recombinant 6His-apo-Me2GlyDH and possible interactors. After washing with 50 mM imidazole, bound 6His-apo-Me2GlyDH and its possible interactors were eluted with 500 mM imidazole, precipitated with acetone, and analyzed by SDS-PAGE. The flavin fluorescence of SDS-PAGE separated proteins was visualized by UV irradiation of the unstained gel soaked in 10% acetic acid. Protein bands were then stained with Coomassie Brilliant Blue. The interactors were searched for by immunoblotting analysis carried out using anti Hsp60 and Hsp10 antibodies. The flavinylation level of Me2GlyDH (inset) was estimated through image analysis as described in Materials and Methods. In (B) matrix and fractions D50 and D200 (100 μg each, lanes 9–11) from ion-exchange chromatography were analyzed by SDS-PAGE and immunoblotting as described in (A).