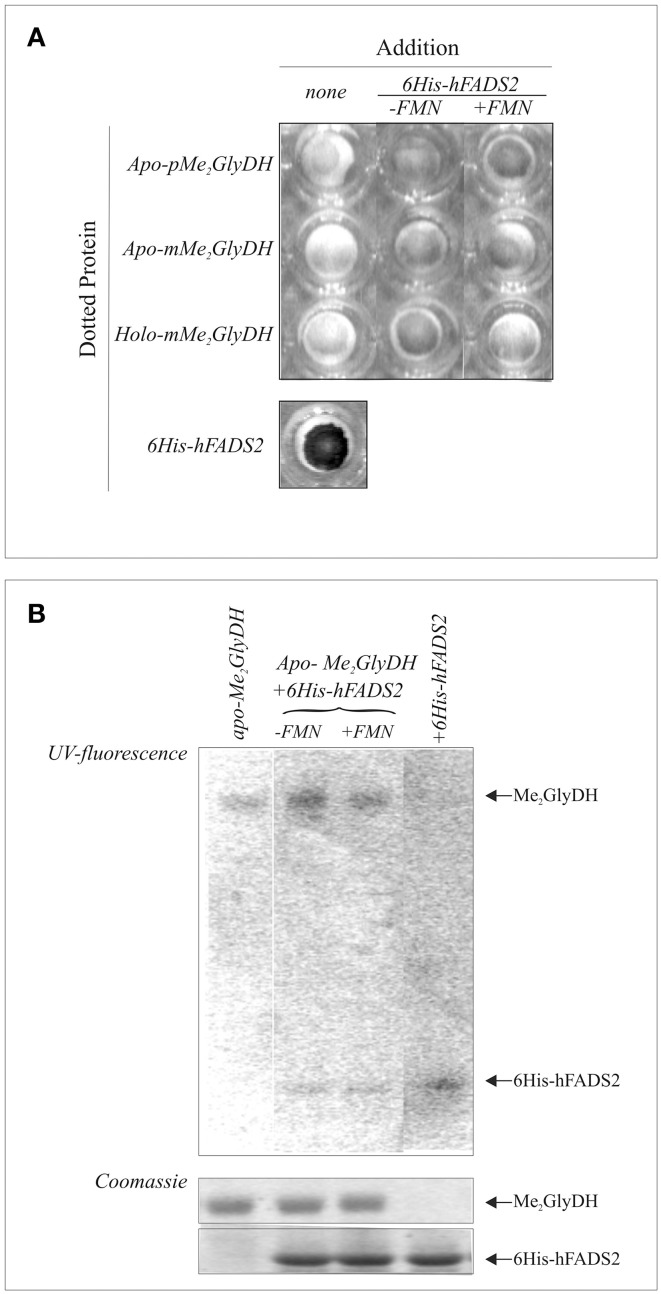
Figure 8.
Evidences of physical interaction and cofactor release from hFADS to the client Me2GlyDH. In (A) purified either precursor (p) or mature (m) form of apo- and holo-Me2GlyDHs (1 μg each) were dotted onto a nitrocellulose membrane and incubated with purified recombinant human 6His-FADS2 in the presence of 5 mM ATP and 5 mM MgCl2. Where indicated 20 μM FMN was added. After 30 min incubation at 37°C the membrane was washed and probed with an anti-hFADS antiserum. In (B) purified recombinant apo-pMe2GlyDH (1 μg) was incubated at 37°C in the presence or absence of recombinant 6His-hFADS2 (3.3 μg) in 40 mM Hepes buffer pH 7.4 containing 5 mM ATP and 5 mM MgCl2. FMN (20 μM) was added where indicated. As a control, 6His-hFADS2 (3.3 μg) was incubated in the same conditions, but in the absence of apo-pMe2GlyDH. After 30 min incubation, protein were denatured with the addition of sample buffer, boiled at 95°C and analyzed by SDS-PAGE. The flavin fluorescence of proteins was visualized by UV irradiation of the unstained gel soaked in 10% acetic acid. Proteins were then stained with Coomassie Brilliant Blue.