Abstract
The immunoglobulin G antitoxoplasma avidity test (Vidas; BioMérieux) is an immunoenzymatic test useful for excluding acute infection after the onset of pregnancy. The avidity index (AI) is the ratio of the signal in a test sample washed with urea, which disrupts low-avidity complexes, to that washed without urea. An AI of >0.3 is taken to mean that infection had occurred more than 4 months ago. The increase of the AI with time and the influence of the different treatments given to pregnant women and their newborns were evaluated. A total of 59 pregnant women (271 sera) and their 60 neonates (199 sera) were tested from 1998 to 2002. There were five groups of women based on the type and duration of treatment given. Thirteen pregnant women (group 1) did not receive any treatment, 15 (group 2), 11 (group 3), and 17 (group 4) women received treatment with spiramycin (9 MIU/day) for 0.5 to 2, 2.5 to 5, and 5.5 to 8 months, respectively, and the last 3 women (group 5) received tritherapy (pyrimethamine-sulfonamide and spiramycin alternatively) for 1.5 to 2.5 months. All of the maternal sera collected in the first 6 months had an AI of <0.30, with a mean of 0.07 (range, 0.01 to 0.21). The increase was slow (0.02/month), and there was no significant difference when comparisons were made between the treatment groups. Neonates with proven maternofetal transmission had an increasing AI, unlike those without transmission. However, long-term therapy with pyrimethamine-sulfonamide, as opposed to treatment with spiramycin alone, was found to slow down the progression of the AI. An AI of >0.2 is sufficient to exclude acute infection in pregnant women. In neonates, it is not of major use to diagnose congenital infection; however, it could be a good indicator of compliance and efficacy of treatment of infected infants.
Toxoplasmosis, caused by Toxoplasma gondii, is widespread in humans and warm-blooded animals. Although usually asymptomatic in immunocompetent humans, toxoplasmosis may cause severe disorders in immunocompromised individuals and in pregnant women because of the high risk of transplacental transmission and the occurrence of abortion or multiple congenital lesions in the fetus (7, 9). In France, specific immunoglobulin G (IgG) and IgM antibody evaluation is mandatory in the first trimester of pregnancy. The presence of toxoplasma-specific IgM at the time of the first blood test is a cause for concern. In certain cases it could either be nonspecific IgM (8, 10, 16-17, 26) or residual IgM from a previous infection (8, 12, 16, 20). In the remaining cases, it could of course be due to an acute infection. To confirm the latter, the presence of an associated elevated IgG level allows a complementary test to be done: the avidity test.
The avidity test (Vidas; BioMérieux, Marcy l'Etoile, France) is of major interest when it is done during the first trimester of pregnancy. It permits dating (an avidity index [AI] of >0.3 means that infection occurred more than 4 months ago) and excludes infection that has occurred after the onset of pregnancy (1, 8, 19). However, some recent studies have shown the limitations and difficulty in interpreting the results of this test (3, 18). Different parameters can modify the increase in the AI during the course of seroconversion. Among these parameters, the sex of the person, pregnancy, immunodepression, and the type of treatment given have been cited (6, 19, 22). Starting treatment early in pregnant women could affect the increase in avidity (22). This hypothesis supported by some authors (6, 19) has not, however, been confirmed by large-scale studies. Moreover, Sensini et al. (22) did not give any information on the type of antibiotics used nor the dose and duration of treatment to validate their conclusions.
In this study, the reliability of the avidity test in a longitudinal follow-up of pregnant women and their newborns for whom seroconversions were accurately dated was evaluated. From these data, we were able to evaluate the influence of the different treatments given and their duration on the increase of the avidity test.
MATERIALS AND METHODS
Diagnosis of seroconversion and antenatal follow-up and treatment.
A total of 59 pregnant women with seroconversion were included in the period from July 1998 to July 2002. The mean age at the time of seroconversion was 28.3 ± 5.3 years (range, 16 to 37 years). Twenty-six women were primiparas, and 33 women were multiparas (21 para 2 and 12 para 3). For each case, more than two sequential serum samples were taken. A total of 271 serum samples were obtained from the 59 women from 1 to 24 months after seroconversion. The follow-up was for more than 6 months in all the cases (6 to 24 months; mean, 12.66 months). It was possible to date infections as accurately as possible because of the obligatory monthly serologic screening in France, and dating was based on the following kinetic criteria. (i) When the first serum sample was negative for both toxoplasma-specific IgM and IgG followed by a seroconversion (positive IgM and IgG) after 1 month, it was taken to mean that infection had occurred between the taking of the two samples (37 cases). (ii) When the first serum sample was IgM positive and the IgG was negative or weakly positive (6 to 100 IU/ml) followed by a twofold or greater increase, it was taken to mean that infection had occurred, at most, 1 month before the first sample (22 cases).
Using these dating criteria, the decision to start treatment was made as follows: (i) no treatment was given if infection had occurred more than 6 weeks before the onset of pregnancy; (ii) spiramycin (9 MIU/day) was given for 2 months if infection had occurred less than 6 weeks before or 4 weeks after the onset of pregnancy, and (iii) treatment with spiramycin (9 MIU/day) was given until the end of pregnancy if infection had occurred more than 5 weeks after the onset of pregnancy. In the latter case, amniotic fluid was collected at 4 weeks or more after the estimated date of infection and never before 18 weeks of gestation. Mouse inoculation and PCR (amplification of the repeated B1 gene of T. gondii) (21) were done with the samples, and if either test was positive, 3-week courses of a tritherapy with sulfadiazine (3 g/day), pyrimethamine (50 mg/day), and folinic acid (50 mg/7 days) were alternated with 3-week courses of spiramycin (9 MIU/day) until the end of pregnancy.
Regardless of the time of onset of infection, all 59 women were distributed in five groups based on the type and duration of treatment they received. Thirteen pregnant women (46 sera, group 1) did not receive any treatment. In seven of these, infection occurred more than 2 months before the onset of pregnancy, and in 6 women, infection was diagnosed at the time of delivery. Fifteen women (59 sera, group 2) received 0.5 to 2 months of treatment with spiramycin (9 MIU/day), 11 women (40 sera, group 3) received 2.5 to 5 months of treatment with spiramycin (9 MIU/day), 17 women (60 sera, group 4) received 5.5 to 8 months of treatment with spiramycin (9 MIU/day), and 3 women (10 sera, group 5), for whom the amniotic fluid was positive, received only one course of the 3-week tritherapy and 3 to 6 weeks of spiramycin (9 MIU/day) because diagnosis was made in late pregnancy.
Pediatric follow-up and treatment.
All neonates were followed up at 2, 4, 6, 9, and 12 months after birth and additionally at 18 and 24 months if congenital toxoplasmosis was proven. Sera were tested from the neonates as well as from the mothers.
A total of 199 samples were obtained from 60 neonates (1 twin pregnancy and 58 single pregnancies). In the 11 cases of congenital toxoplasmosis, two alternative treatments were begun according to the protocol used by the obstetric unit: spiramycin alone (150 MIU/kg of body weight/day) for 1 year for 4 asymptomatic patients and tritherapy for 5 asymptomatic and 2 symptomatic patients. The tritherapy corresponded to 3-week courses of sulfadiazine (75 mg/kg/day), pyrimethamine (3 mg/kg every 3 days), and folinic acid (50 mg/7 days), alternating with 5-week courses of spiramycin (150 MIU/kg/day) for 1 year. If the compliance of the latter protocol was difficult, the following tritherapy was used beginning at 2 months up to 1 year of age: pyrimethamine-sulfadoxine (1.25 mg and 25 mg, respectively, per kg per 10 days) (Fansidar) and folinic acid (50 mg/7 days) (5 of 7 patients).
Serological tests.
Toxoplasma-specific IgG antibodies were detected by two commercial enzyme immunoassays (Access Toxo-IgG [Beckman Coulter France, Roissy, France] and Vidas Toxo-IgG [BioMérieux]) and by in-house immunofluorescent assays (IFA), where the cutoff of 8 IU/ml for positivity was determined by using international standards (2). A grey zone between 4 and 8 IU/ml was included because of the interreader subjectivity of the IFA. The following values were considered negative, equivocal, and positive, respectively, for the two commercial tests according to the manufacturers recommendations: Access Toxo-IgG, <4.0, 4.0 to 6.0, and >6.0 IU/ml; Vidas Toxo-IgG, <4.0, 4.0 to 8.0, and >8.0 IU/ml.
Toxoplasma IgM antibodies were detected by enzyme immunoassay (Access Toxo-IgMII; Beckman Coulter France), by in-house IFA (2), and by immunosorbent agglutination assays (IgM ISAgA; BioMérieux). The results are quantitative and are expressed in arbitrary units. The following titers were considered negative, equivocal, and positive, respectively, for the following tests: Access Toxo-IgM, <0.80, 0.80 to 1.00, and >1.00 signal/cutoff (S/CO) ratio; IFA, <1/20, 1/20, and ≥1/40 inverse of dilution; immunosorbent agglutination assays for obstetrical samples, ≤4+, 5+ to 8+, and 9+ to 12+. For pediatric samples, ≥3+ was considered significant.
The IgG avidity test was performed as previously described by Pelloux et al. (19) with an immunoenzymatic commercial kit (Vidas Toxo IgG avidity kit; BioMérieux) and by following the instructions of the manufacturer. This test is performed by the fully automated Vidas machine, which also automatically executes the calculation and interpretation of results. The AI is the ratio of the signal in the test sample washed with 6 M urea, which disrupts low-avidity complexes, to that washed without 6 M urea. Interpretation was as follows: <0.200, low avidity; 0.200 to 0.300, borderline avidity; >0.300, high avidity. The manufacturer states that, with this method, a high-avidity result enables exclusion of a recently acquired infection during the last 4 months before the sample was taken.
Statistical analysis.
Spearman's rank correlation test was used to determine the association between the AI and the time elapsed between infection and sampling.
A statistical assessment of differences of the mean number of months before reaching the threshold level for the avidity test in the different treatment groups was estimated by analysis of variance (ANOVA) (Fisher-Snedecor test). A P value of <0.05 was considered significant.
RESULTS
Serological follow-up of pregnant women. (i) Increase in AI.
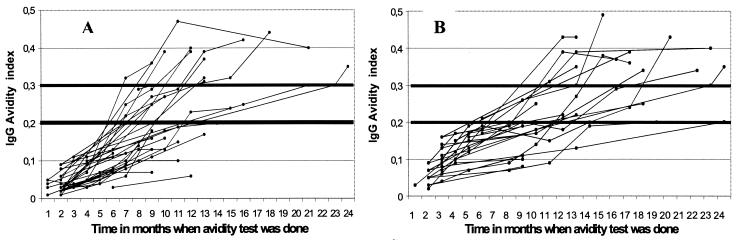
We were able to do 215 avidity tests with the 271 postseroconversion serum samples. The remaining 56 samples had insufficient IgG titers (<15 IU/ml) to do the test (beginning of seroconversion). A total of 103 sera were collected in the first 6 months after infection, 73 samples were collected between 6 and 12 months after infection, 30 sera were collected between 12 and 18 months, and 9 samples were taken between 18 and 24 months after infection. All of the sera collected in the first 6 months had an AI inferior to the company threshold of 0.30, with a mean of 0.07 (range, 0.01 to 0.21). The increase was slow (mean increase of AI, 0.02 per month), but the correlation between the increase in AI and time of testing was significant (r = 0.85, Spearman's Rank test) (Fig. 1). The rate of increase was variable for the different cases. Thus, the avidity threshold of 0.2 was reached between 6 and 24 months after infection (mean, 11.49 ± 3.90 months), and the threshold of 0.3 was reached from 7 to >24 months after infection (mean, 14.20 ± 4.80 months) (Fig. 1).
FIG. 1.
Increase of the AI in 59 pregnant women followed up after accurately dated seroconversion (total number of avidity tests, 215; correlation coefficient of Spearman, r = 0.85, y = 0.020x + 0.010). (A) Infection had occurred between the two samples (real seroconversion) (37 patients); (B) infection had occurred at most 1 month before the first sample (positive IgM and negative or weakly positive IgG followed by a twofold or greater increase) (22 patients) (no difference between the two groups).
As the data suggested that the company threshold was too high, the receiver operating characteristic (ROC), with a range of cutoffs of 0.10, 0.12, 0.15, 0.20, 0.25, and 0.30, was calculated (Table 1). The cutoff value for 100% exclusion of acute infection (<4 months), as determined from the ROC analysis, corresponded to an AI of 0.20. This choice of threshold allowed the exclusion of acute infection in 72 of 141 (51.1%) sera without further testing, compared to the company threshold of 0.30, which allowed the exclusion of acute infection in only 39 of 141 (27.7%) sera (Table 1).
TABLE 1.
ROC analysis of AI for diagnosis of chronic toxoplasmosisa
| AI | Acute infection (≤4 mo; n = 74)
|
Chronic infection (>4 mo; n = 141)
|
||
|---|---|---|---|---|
| No. (%) of cases ≥ threshold | No. (%) of cases < threshold | No. (%) of cases ≥ threshold | No. (%) of cases < threshold | |
| 0.10 | 14 (18.9) | 60 (81.1) | 119 (84.4) | 22 (15.6) |
| 0.12 | 7 (9.5) | 67 (90.5) | 110 (78) | 31 (22) |
| 0.15 | 4 (5.4) | 70 (94.6) | 97 (68.8) | 44 (31.2) |
| 0.17 | 1 (1.4) | 73 (98.6) | 89 (63.1) | 52 (36.9) |
| 0.20 | 0 (0) | 74 (100)b | 72 (51.1)c | 69 (48.9) |
| 0.25 | 0 (0) | 74 (100) | 55 (39) | 86 (61) |
| 0.30 | 0 (0) | 74 (100) | 39 (27.7) | 102 (72.3) |
Specificity and sensitivity were calculated for different thresholds of the AI (0.30, as given by the manufacturer, and 0.10, 0.12, 0.15, 0.17, 0.20, and 0.25). The cutoff value for 100% exclusion of acute infection (<4 months) corresponded to an AI of 0.20.
Specificity at the threshold of 0.2.
Sensitivity at the threshold of 0.2.
(ii) Influence of antitoxoplasma treatment on the increase of the AI.
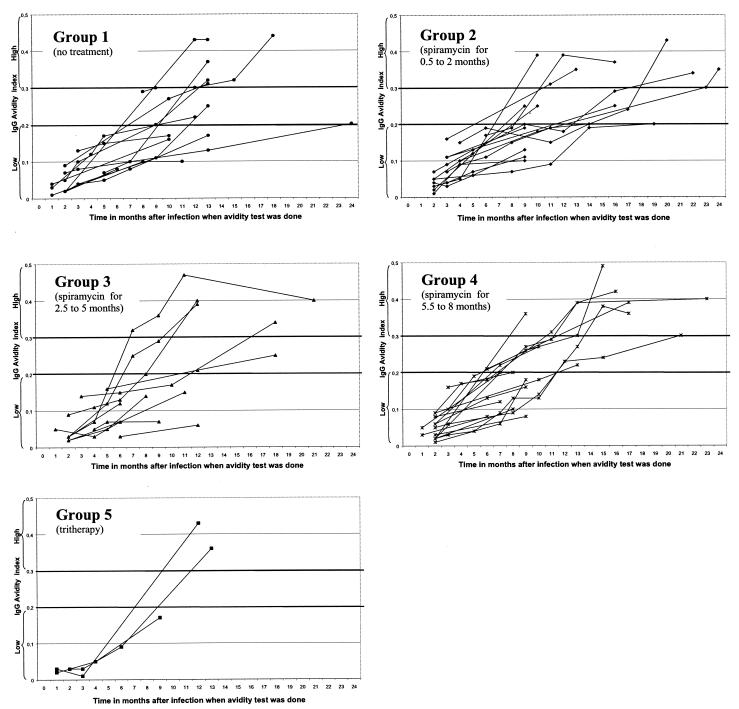
The increase of the AI over time as a function of the type and duration of treatment is shown in Fig. 2. As described above, there is a high interindividual variability in the kinetics of the AI over time. There was no significant difference in this variability when comparisons were made between the five treatment groups. Thus, the mean numbers of months required to reach the threshold AI of 0.2 were 12.62 ± 4.96, 13.00 ± 3.29, 10.40 ± 4.72, 9.45 ± 2.46, and 12.50 ± 0.71 months for groups 1, 2, 3, 4, and 5, respectively (Fisher Snedecor test, P = 0.19), and those required to reach an AI of 0.3 were 14.00 ± 5.14, 16.33 ± 5.96, 12.25 ± 4.50, 14.14 ± 3.98, and 12.50 ± 0.71 months for groups 1, 2, 3, 4, and 5, respectively (Fisher Snedecor test, P = 0.72).
FIG. 2.
Increase of the AI in pregnant women followed up as a function of the duration and type of antitoxoplasma treatment. Group 1, no treatment (13 patients, 46 sera); group 2, spiramycin (9 MIU/day) for 0.5 to 2 months (15 patients, 59 sera); group 3, spiramycin (9 MIU/day) for 2.5 to 5 months (11 patients, 40 sera); group 4, spiramycin (9 MIU/day) for 5.5 to 8 months (17 patients, 60 sera); group 5, tritherapy for 1.5 to 2.5 months (sulfadiazine [3 g/day], pyrimethamine [50 mg/day], and folinic acid [50 mg/7 days]) alternated with 3-week courses of spiramycin (9 MIU/day) (3 patients, 10 sera) (for cutoff value of 0.2, P = 0.19 [ANOVA Fisher Snedecor test]; for cutoff value of 0.3, P = 0.72 [ANOVA Fisher Snedecor test]).
Serological follow-up of neonates. (i) Increase in AI in pediatric patients.
From the 60 neonates monitored, 199 serum samples were obtained. Of these, it was possible to do the avidity test for only 105 because the IgG titer was too low to do the test in the remaining 94 (there was a rapid decrease in IgG titer in children who were not infected).
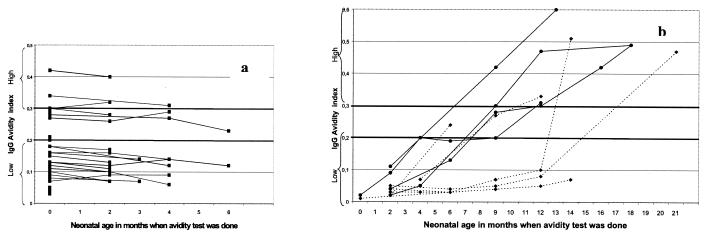
Eleven of 60 children (46 of 48 sera for which the avidity test was practicable) had congenital toxoplasmosis diagnosed by parasitological and serological tests. The remaining 49 children (59 of 151 sera for which the avidity test was practicable) either had no maternofetal transmission proven after 2 serology-negative serum samples and a follow-up at ≥1 year (29 children) or were considered indeterminate because some are still being followed up and others were lost during follow-up (20 children). In all 49 patients, only minimal change in the AI was observed over time (range, 0.00 to 0.06). In contrast, among the 11 congenital toxoplasmosis patients, the change was significant (range, 0.04 to 0.49) (Fig. 3).
FIG. 3.
Increase of the AI in 60 neonates followed up for congenital toxoplasmosis. (a) 49 neonates (59 sera) in whom maternofetal transmission was either negative or indeterminate; (b) 11 neonates (46 sera) in whom maternofetal transmission was proven. Dotted lines indicate neonates treated with tritherapy. Solid lines indicate neonates treated with spiramycin alone.
(ii) Influence of antitoxoplasma treatment on the increase of the AI.
Of the 11 congenital toxoplasmosis patients, for which 46 sera were tested, 1 patient had severe infection and died in the third month (B. Lecomte, H. Patural, C. Paricio, P. Flori, R. Tran Manh Sung, and G. Teyssier, submitted for publication). Among the remaining 10 patients, the increase of the AI was different depending on the type of treatment given. It is interesting that the increase in the AI was much faster in children treated with only spiramycin. Thus, at the end of 1 year of treatment, all 4 children treated with spiramycin reached the threshold of 0.3. However, of the 6 children treated with tritherapy, only 1 reached the threshold of 0.3 and 1 was lost during follow-up.
DISCUSSION
Differentiation between a recently acquired T. gondii infection from a chronic one is important in pregnant women. Thus, a high AI permits us to exclude a recent seroconversion and to reassure up to 67% of pregnant women presenting with elevated antitoxoplasma IgM levels in the first trimester of pregnancy (1, 6, 8, 11, 15). The Vidas (BioMérieux) avidity test has been standardized and optimized for this purpose (19). Due to its automatization, it is reliable and reproducible. This test should never be used in isolation but always associated with a technique that quantifies specific IgG and IgM. Thus, it permits us to differentiate the presence of nonspecific or residual IgM from that present due to a recent infection (8, 10, 12, 16-17, 20, 26). It does not allow us to make a decision regarding interruption of pregnancy, but on the other hand, it permits us to reassure a large number of patients at the beginning of pregnancy when the AI is >0.3. This test has been developed as a routine test in many European countries (3, 11, 19) and in South America, where it is commercialized. In addition, it has been evaluated in the United States (1, 15, 18). The obligatory monthly serological screening of pregnant women in France has made it possible for us to evaluate the increase of the AI with time and to see the effect of treatment on the results of this test. As opposed to previous studies, all of the samples in our study were obtained from patients with accurately dated seroconversions followed up longitudinally. We have evaluated the kinetics of the test solely in pregnant women to avoid the variations associated with different parameters, such as sex and immunodepression (6, 19).
The increase in the AI was slow; the average time required to reach the threshold of 0.3 was 14.2 months. No women reached this threshold either at 4 months or at 6 months after seroconversion. Only one of the 59 reached it in less than 9 months. It is therefore justifiable to decrease the threshold for exclusion of a recent infection of less than 4 months to 0.2 and use the company threshold of 0.3 for exclusion of infections which have occurred more than 6 months back. This leaves a margin of safety of 3 months so as not to miss the rare but possible fetal transmission following periconceptional infection (4, 24, 25).
In view of the slow increase of the AI, treatment with spiramycin started early could be a limiting factor. To prove this, the kinetics of the avidity test in women treated with those who were not treated were compared. However, no significant difference was found between the different treatment groups. These data confirm the results of the study by Jenum et al. (11), the only other study with a longitudinal follow-up. As for the tritherapy, the number of patients and the duration of treatment were too small to evaluate the influence on the kinetics of the avidity test. This needs to be studied with a larger number of patients.
Concerning the pediatric follow-up, there is only one other study which has evaluated the avidity test (14). At birth, the AI of the neonates was similar to that of their mothers. However, during the follow-up, at 2, 4, 6, 9, and 12 months, two different profiles emerged: that of the patients with proven maternofetal transmission and that of those without transmission. In the absence of maternofetal transmission, the AI did not increase and became impracticable due to the rapid decrease in the concentration of the transmitted maternal antibodies. In the presence of maternofetal transmission, a significant increase of the AI in 4 patients treated with only spiramycin confirmed the transmission. The increase of the AI was faster than that of their mothers (data not shown). This increase, however, required a delay of 4 months or more in neonates. At this age, almost all (96 to 98%) infected neonates were already diagnosed by serological and parasitological techniques (21, 23). This delay is therefore a drawback in the use of this test to diagnose congenital infection, except in the rare cases where the decrease of the IgG titer is slow and in the absence of antenatal or postnatal proof of infection. In contrast, it is interesting that long-term tritherapy for neonates was found to slow down the progression of the AI. Among the 6 patients that took the tritherapy, for 4 patients the AI did not increase during the full course of the treatment. For 1 patient, the AI reached 0.3 but the treatment regimen was not followed correctly (only three courses of the 3-week treatment in 1 year), and the other patient was lost during the follow-up. The absence of increase in the IgG avidity in neonates treated with tritherapy along with a stable or decreasing concentration of IgG (13) could be proof of the efficacy of treatment, which could limit the contact between the parasite and the immune system. This test could therefore be particularly useful for detecting inefficient treatment or poor follow-up, as compliance is difficult with infants due to the long duration and poor tolerance of the tritherapy (5, 13). These findings may confirm the suggestion of different investigators (5, 13, 23), who propose giving only tritherapy even for asymptomatic cases.
To conclude, the Vidas (BioMérieux) avidity test was found to be reliable. It was demonstrated that the increase in the IgG avidity is slow, and therefore, an AI superior to 0.2 could safely be used to exclude an acute infection of less than 4 months in pregnant women instead of 0.3. Treatment with spiramycin had no influence on the increase of the AI in pregnant women. In neonatal follow-up, the avidity test is not of major use for diagnosing congenital infection; however, it could be a good indicator of compliance and the efficacy of treatment in infected infants.
Acknowledgments
We are indebted to various individuals, including Martine Wallon, Philippe Gay, and Georges Belot and their laboratory team, who provided us with some missing sera in the course of the pediatric follow-up. We acknowledge the staff of the Laboratory of Parasitology for skillful technical assistance.
REFERENCES
- 1.Alvarado-Esquivel, C., S. Sethi, K. Janitschke, H. Hahn, and O. Liesenfeld. 2002. Comparison of two commercially available avidity tests for toxoplasma-specific IgG antibodies. Arch. Med. Res. 33:520-523. [DOI] [PubMed] [Google Scholar]
- 2.Ambroise-Thomas, P., J. P. Garin, and A. Rigaud. 1966. Amélioration de la technique d'immuno-fluorescence par l'emploi de contre-colorants. Application aux toxoplasmes. Presse Med. 74:2215-2216. [PubMed] [Google Scholar]
- 3.Barberi, A., A. Gistri, F. Cappelletti, and I. Giordano. 2001. Diagnostic value of IgG avidity in Toxoplasma infection. Comparison of 3 commercial kits. J. Infect. Dis. 184:944-946. [DOI] [PubMed] [Google Scholar]
- 4.Chemla, C., I. Villena, D. Aubert, P. Hornoy, D. Dupouy, B. Leroux, J. P. Bory, and J. M. Pinon. 2002. Preconception seroconversion and maternal seronegativity at delivery do not rule out the risk of congenital toxoplasmosis. Clin. Diagn. Lab. Immunol. 9:489-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couvreur, J. 1999. Problems of congenital toxoplasmosis. Evolution over four decades. Presse Med. 28:753-757. [PubMed] [Google Scholar]
- 6.Cozon, G. J. N., J. Ferrandiz, H. Nebhi, M. Wallon, and F. Peyron. 1998. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 17:32-36. [DOI] [PubMed] [Google Scholar]
- 7.Desmonts, G., and J. Couvreur. 1974. Congenital toxoplasmosis: a prospective study of 378 pregnancies. N. Engl. J. Med. 290:1110-1116. [DOI] [PubMed] [Google Scholar]
- 8.Flori, P., J. Hafid, H. Raberin, H. Patural, M. N. Varlet, and R. Tran Manh Sung. 2002. Relevance of the new test Access Toxo IgM (II) in the serological interpretation of toxoplasmosis in pregnancy. Ann. Biol. Clin. 60:65-72. [PubMed] [Google Scholar]
- 9.Foulon, W., I. Villena, B. Stray-Pedersen, A. Decoster, M. Lappalainen, J. M. Pinon, P. A. Jenum, K. Hedman, and A. Naessens. 1999. Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children's sequelae at age 1 year. Am. J. Obstet. Gynecol. 180:410-415. [DOI] [PubMed] [Google Scholar]
- 10.Hofgartner, W. T., S. R. Swanzy, R. M. Bacina, J. Condon, M. Gupta, P. E. Matlock, D. L. Bergeron, J. J. Plorde, and T. R. Fritsche. 1997. Detection of immunoglobulin G (IgG) and IgM antibodies to Toxoplasma gondii: evaluation of four commercial immunoassay systems. J. Clin. Microbiol. 35:3313-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenum, P. A., B. Stray Pedersen, and A. G. Gundersen. 1997. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 35:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenum, P. A., B. Stray-Pedersen, K. K. Melby, G. Kapperud, A. Whitelaw, A. Eskild, and J. Eng. 1998. Incidence of Toxoplasma gondii infection in 35,940 pregnant women in Norway and pregnancy outcome for infected women. J. Clin. Microbiol. 36:2900-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieffer, F., P. Thulliez, A. Brezin, R. Nobre, S. Romand, E. Yi-Gallimard, M. Voyer, and J. F. Magny. 2002. Treatment of subclinical congenital toxoplasmosis by sulfadiazine and pyrimethamine continuously during 1 year: apropos of 46 cases. Arch. Pediatr. 9:7-13. [DOI] [PubMed] [Google Scholar]
- 14.Lappalainen, M., M. Koskiniemi, V. Hiilesmaa, P. Ammala, K. Teramo, P. Koskela, M. Lebech, K. O. Raivio, and K. Hedman. 1995. Outcome of children after maternal primary Toxoplasma infection during pregnancy with emphasis on avidity of specific IgG. Pediatr. Infect. Dis. J. 14:354-361. [DOI] [PubMed] [Google Scholar]
- 15.Liesenfeld, O., J. G. Montoya, S. Kinney, C. Press, and J. S. Remington. 2001. Effect of testing for IgG avidity in the diagnosis of Toxoplasma gondii infection in pregnant women: experience in a US reference laboratory. J. Infect. Dis. 183:1248-1253. [DOI] [PubMed] [Google Scholar]
- 16.Liesenfeld, O., J. G. Montoya, N. J. Tathineni, M. Davis, B. W. Brown, Jr., K. L. Cobb, J. Parsonnet, and J. S. Remington. 2001. Confirmatory serologic testing for acute toxoplasmosis and rate of induced abortions among women reported to have positive toxoplasma immunoglobulin M antibody titers. Am. J. Obstet. Gynecol. 184:140-145. [DOI] [PubMed] [Google Scholar]
- 17.Liesenfeld, O., C. Press, J. G. Montoya, R. Gill, J. L. Isaac-Renton, K. Hedman, and J. S. Remington. 1997. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J. Clin. Microbiol. 35:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montoya, J. G., O. Liesenfeld, S. Kinney, C. Press, and J. S. Remington. 2002. Vidas test for avidity of Toxoplasma-specific immunoglobulin G for confirmatory testing of pregnant women. J. Clin. Microbiol. 40:2504-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelloux, H., E. Brun, G. Vernet, S. Marcillat, M. Jolivet, D. Guergour, H. Fricker-Hidalgo, A. Goullier-Fleuret, and P. Ambroise-Thomas. 1998. Determination of anti-Toxoplasma gondii immunoglobulin G avidity: adaptation to the Vidas system (bioMérieux). Diagn. Microbiol. Infect. Dis. 32:69-73. [DOI] [PubMed] [Google Scholar]
- 20.Pelloux, H., H. Fricker-Hidalgo, A. Goullier-Fleuret, and P. Ambroise-Thomas. 1997. Detection of anti-Toxoplasma immunoglobulin M in pregnant women. J. Clin. Microbiol. 35:2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romand, S., M. Wallon, J. Franck, P. Thulliez, F. Peyron, and H. Dumon. 2001. Prenatal diagnosis using polymerase chain reaction on amniotic fluid for congenital toxoplasmosis. Obstet. Gynecol. 97:296-300. [DOI] [PubMed] [Google Scholar]
- 22.Sensini, A., S. Pascoli, D. Marchetti, R. Castronari, M. Marangi, G. Sbaraglia, C. Cimmino, A. Favero, M. Castelletto, and A. Mottola. 1996. IgG avidity in the serodiagnosis of acute Toxoplasma gondii infection: a multicenter study. Clin. Microbiol. Infect. 2:25-29. [DOI] [PubMed] [Google Scholar]
- 23.Villena, I., C. Chemla, D. Aubert, F. Foudrinier, and J. M. Pinon. 2003. Congenital toxoplasmosis: neonatal biological diagnosis and surveillance. Arch. Pediatr. 10:39-41. [PubMed] [Google Scholar]
- 24.Villena, I., C. Chemla, C. Quereux, D. Dupouy, B. Leroux, F. Foudrinier, and J. M. Pinon. 1998. Prenatal diagnosis of congenital toxoplasmosis transmitted by an immunocompetent woman infected before conception. Prenat. Diagn. 18:1079-1081. [PubMed] [Google Scholar]
- 25.Vogel, N., M. Kirisits, E. Michael, H. Bach, M. Hostetter, K. Boyer, R. Simpson, E. Holfels, J. Hopkins, D. Mack, M. B. Mets, C. N. Swisher, D. Patel, N. Roizen, L. Stein, M. Stein, S. Withers, E. Mui, C. Egwuagu, J. Remington, R. Dorfman, and R. McLeod. 1996. Congenital toxoplasmosis transmitted from an immunologically competent mother infected before conception. Clin. Infect. Dis. 23:1055-1060. [DOI] [PubMed] [Google Scholar]
- 26.Wilson, M., J. S. Remington, C. Clavet, G. Varney, C. Press, and D. Ware. 1997. Evaluation of six commercial kits for detection of human immunoglobulin M antibodies to Toxoplasma gondii. J. Clin. Microbiol. 35:3112-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]