Table 3.
Anti-proliferative activity manifested by substituted biphenyl derivatives.
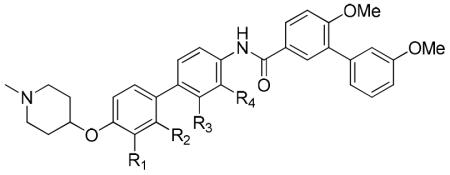
| Entry | R1 | R2 | R3 | R4 | SKBr3 (IC50, μM) |
MCF-7 (IC50, μM) |
|---|---|---|---|---|---|---|
| 8i | H | H | H | H | 0.47±0.06 | 0.71±0.02 |
| 29a | Me | H | H | H | 0.83±0.03 | 1.69±0.08 |
| 29b | H | Me | H | H | 1.18±0.11 | 1.21±0.03 |
| 29c | H | H | Me | H | 0.97±0.01 | 1.57±0.56 |
| 29d | H | H | H | Me | 2.47±0.39 | 1.43±0.35 |
| 29e | OMe | H | H | H | 0.68±0.13 | 1.32±0.08 |
| 29f | H | OMe | H | H | 1.41±0.35 | 1.35±0.16 |
| 29g | H | H | OMe | H | 0.90±0.08 | 1.50±0.08 |
| 29h | H | H | H | OMe | 3.92±0.21 | 1.22±0.04 |
| 29i | Cl | H | H | H | 1.84±0.57 | 1.48±0.12 |
| 29j | H | Cl | H | H | 1.28±0.14 | 1.48±0.33 |
| 29k | H | H | Cl | H | 2.21±0.18 | 3.44±0.21 |
| 29l | H | H | H | Cl | 4.29±0.65 | 1.80±0.19 |
| 34a | NO2 | H | H | H | 2.07±0.17 | 1.23±0.25 |
| 34b | H | NO2 | H | H | 1.18±0.15 | 1.30±0.12 |
| 34c | H | H | NO2 | H | 2.48±0.77 | 3.32±0.25 |
| 34d | H | H | H | NO2 | 3.40±0.14 | 1.15±0.01 |
| 38a | NH2 | H | H | H | 2.23±0.49 | 5.95±1.22 |
| 38b | H | NH2 | H | H | 2.13±0.06 | 1.76±0.37 |
| 38c | H | H | NH2 | H | 3.90±0.18 | 2.07±0.23 |
| 38d | H | H | H | NH2 | 3.21±0.45 | 2.25±0.49 |
| 39a | NHAc | H | H | H | 2.66±0.76 | 1.84±0.43 |
| 39b | H | NHAc | H | H | 3.39±0.66 | 1.36±0.23 |
| 39c | H | H | NHAc | H | 2.52±0.26 | 4.66±0.49 |
| 39d | H | H | H | NHAc | 3.51±0.56 | 1.66±0.59 |
