Abstract
Purpose
Breast Cancer Resistance Protein (BCRP) belongs to the family of efflux transporters involved in drug efflux leading to drug resistance. The objective of this study was to explore physical barriers for ocular drug absorption and to verify the presence and possible role of BCRP as a bar-rier for ocular drug resistance.
Methods
Transfected human corneal epithelial cells (SV40-HCEC) were selected as an in vitro model for corneal epithelium with MDCKII-BCRP as positive control. [3H]-Mitoxantrone ([3H]-MTX), which is a proven substrate for organic anion transporter like BCRP, was selected as a model drug for functional expression studies. Fumetremorgin C (FTC), a known specific inhibitor for BCRP and GF120918, an inhibitor for BCRP and P-gp, were added to inhibit BCRP-mediated efflux. PGP-4008, a specific inhibitor of P-gp was used to delineate the contribution of P-gp. The mRNA extracted from cells was used for RT-PCR analysis and gene expression. Membrane fractions of SV40-HCEC and MDCKII-BCRP were used for immunoprecipitation followed by Western blot analysis.
Results
Efflux was inhibited significantly in the presence of FTC and GF120918. Dose-dependent inhibition of efflux by BCRP was noticed in SV40-HCEC and MDCKII-BCRP in the presence of FTC and GF120918, and the efflux was ATP-dependent. The metabolic inhibitor, 2,4-DNP, significantly inhibited efflux. No pH-dependent efflux was noticed except at pH 5.5. RT-PCR analysis indicated a unique and distinct band at ~429 bp, corresponding to BCRP in SV40-HCEC and MDCKII-BCRP cells. Western Blot analysis indicated a specific band at ~70 kDa in the membrane fraction of SV40-HCEC and MDCKII-BCRP cells.
Conclusions
We have demonstrated the expression of BCRP in human corneal epithelial cells and, for the first time, demonstrated its functional activity leading to drug efflux. RT-PCR and Western blot analysis further confirmed this finding.
INTRODUCTION
ATP-binding cassette (ABC) proteins belong to a super family of transmembrane transporters that use the energy obtained from ATP hydrolysis to transport their substrate drug molecules across biological membranes.1 P-glycoprotein (P-gp; MDR1)2 and multidrug resistance associated proteins (MRP),3,4 are drug resistance proteins and are considered to be a major barrier for ocular drug delivery. MDR1 and MRP2, which are present on the cornea, can play a collective role in the efflux of drug molecules.3 Apart from MRP2, human corneal cells have also been shown to express MRP1 and MRP3.4,5 Erythromycin,3 quinolones (ciprofloxacin, grepafloxacin),5,6,7 steroids, sulfated steroids, and estradiol 17β-glucuronide,8,9,10 are widely employed in ocular therapy and are excellent substrates for various efflux pumps. P-gp expression encoded by the MDR1 gene and expression of the MRP gene was shown to correlate with a reduction in intracellular drug concentrations.11,12 As stated in Mao Q et al. (2005),11 “recently, apart from P-gp and MRP, several human ATP-binding cassette transporters with an important role in drug efflux have been discovered. One of them is a novel protein known as the breast cancer resistance protein (BCRP),13 or mitoxantrone-resistance protein (MXR),14 or placenta-specific ABC protein (ABCP),15 and was identified independently by three research labs.16 BCRP, with a molecular weight of ~70 kDa, is a major efflux pump involved in drug resistance.16 Unlike P-glycoprotein and MRP, which are arranged in two repeated halves, BCRP consists of one nucleotide-binding and one membrane-spanning domain and is regarded as a half transporter.”17 BCRP is classified under the new branch, subfamily G, belonging to the large ABC transporter family.16 ABCG1, which is a human homologue of Drosophila white gene, is the founding member of the ABCG family.16,17 BCRP is categorized as the second member of the subfamily G and, hence, was designated ABCG2.16
As indicated by Mao et al. (2005), “Comparison of ABCG protein sequences with that of P-gp and MRP1 revealed that, unlike P-gp and MRP1, which are organized in two repeated halves, all ABCG proteins are half transporters that are composed of a single nucleotide-binding domain (NBD) followed by one membrane-spanning domain (MSD). There is increasing evidence to suggest that ABCG proteins may operate as either homodimers or heterodimers.”18,19
The literature suggests that BCRP can efflux conjugated organic anions in transport studies performed on plasma membrane vesicles.16 BCRP was found to transport estrone-3-sulfate (E1S) with a Km value of ~10 μM.16,20,21 With its broad substrate specificity, along with sulfated conjugates, BCRP was also found to transport E217βG and DNP-SG, which are glucuronide conjugates.16,22 The affinity for sulfated conjugates was found to be enhanced compared to GSH and glucoronide conjugates.16,22 E1S and DHEAS represent the major estrogens that are manufactured and eliminated by syncytiotrophoblasts of placenta in the mother and were the first substrates of BCRP identified.16 BCRP was also found to transport unconjugated organic anions.16 An interesting study by Umemoto et al. (2005) revealed that basal limbal epithelial cells of the rat eye express BCRP significantly. Also, side population stem cells, which help in the regeneration of corneal epithelium, express BCRP, but at a relatively very low level compared to limbal side population cells.23
Therefore, the main objective of this study was to determine if human corneal epithelial cells express a major ATP-binding efflux transporter, BCRP, that is commonly expressed on the apical side of the cell membrane16 and examine its role in drug efflux. Efflux of [3H]−MTX in SV40-HCEC and MDCKII-BCRP, use of FTC and GF120918 as specific inhibitors of BCRP, RT-PCR data for gene expression and immunoprecipitation, followed by Western blot analysis for protein expression, were applied to confirm the presence of BCRP in SV40-HCEC.
MATERIALS AND METHODS
Fumtremorgin C (FTC), a specific inhibitor for BCRP,16 2-deoxy-D-glucose, and sodium azide were obtained from Sigma Chemical Co. (St. Louis, MO, USA). GF120918, a dual inhibitor of BCRP and P-gp,17 was a generous gift from Glaxo Wellcome (Research Triangle, NC, USA). PGP-4008, a specific P-gp inhibitor47 and poly-D-lysine were obtained from Fischer Science (Pittsburgh, PA, USA). [3H]-E17βg was obtained from Moravek Biochemical's and Radiochemicals (Brea, CA, USA). Twenty-four well culture plates were purchased from Midwest Scientific and were employed for transport studies (St. Louis, MO, USA).
Cell Culture
Culture of Human Corneal Epithelial Cells (SV40-HCEC)
The SV40-immortalized human corneal epithelial cell line was obtained as a gift from Araki-Sasaki and colleagues.24 Cells were cultured based on published protocol with minor modification.25 Mycoplasma-free SV40-HCEC were grown at 37°C, humidified in a 5% CO2/95% air atmosphere in a culture medium containing 50% of Dulbecco's Modified Eagle's Medium (DMEM) and 50% of Ham's nutrient mixture F-12 (from Gibco, Paisley, UK), (v/v) fetal bovine serum, 15% as supplement (GIBCO), antibiotic solution (gentamycin (30 μg/ml) from Gibco) and sodium bicarbonate (1.86 g/l). Cells were harvested using trypsin-EDTA (Gibco).
MDCKII-BCRP Cells
MDCKII-BCRP cells were transfected with a viral vector having the human BCRP gene, which are known to overexpress BCRP on the apical side of the cell membrane.26 They were obtained as a generous gift from Dr. Peit Borst (Netherlands Cancer Institute, Amsterdam). A well-established and published protocol was used to culture the cells. As indicated in earlier publications from our laboratory, “Cells were maintained in DMEM supplemented with 10% calf serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 20 mmol/l HEPES, pH 7.4. Cells were plated at a density of 100,000/cm2 in 12-well tissue culture treated plastic plates. MDCKII-BCRP cells were incubated at 37°C in an atmosphere of 5% CO2 and 95% humidity in air and were allowed to grow for 5–8 days.”27
Human Gingival Fibroblast Cells (HGF-1 Cells)
Human gingival fibroblast cells were procured from American Type Culture Collection (Manassas, VA, USA). Cells were cultured using the DMEM supplemented medium we employed for culturing the MDCKII-BCRP cells. Similar culture conditions were maintained.
Transport Experiments
To determine functional activity of BCRP in corneal epithelial cells, transport studies were performed with MTX. Transport studies were performed based on the protocol established by Schuetz et al. (1999), with minor modifications.28 Monolayers of MDCKII-BCRP and SV40-HCEC cultured in 12-well tissue culture plates (Fisher Scientific, Pittsburg, PA, USA) were employed. As reported previously, the addition of 10 mM sodium azide and 10 mM 2-deoxy-D-glucose to cell monolayers reduced intracellular ATP by >90% in the first 5 min of incubation, but did not affect cell viability.29 The principle behind this study involves preloading drug into cells by deactivating the efflux transporter (ATP depleting conditions), washing, and then reactivating the transporter with a medium containing glucose.29 Transport kinetics of BCRP were observed with and without inhibitors.
Cells were incubated for 60 min with 3H-bis (POM) PMEA (1μCi) and 3H-acyclovir (1μCi) in the presence of 10 mM sodium azide and 2-deoxy-D-glucose. Following a 1-hr incubation period under normal and ATP-depleting conditions, cell viability was determined by the trypan blue exclusion test. Cells were examined for viability (unstained) and nonviability/cytotoxicity (stained). A hemocytometer (Hausser Scientific, Horsham, PA, USA) was used to count the cells. After the incubation, the drug solution was removed from the wells, and the cells were washed with 0.16 M NaCl and subsequently incubated for 60 min with Hanks buffered saline solution (HBSS) with glucose, pH 7.2, and HBSS with glucose incorporating the inhibitors. Preparation of HBSS with inhibitors is described in the next section. The supernatant was collected and the radioactivity measured with a Beckmann scintillation counter (Beckman Coulter, Inc., Fullerton, CA, USA). The amount of radioactivity in the supernatant represents the amount of drug effluxed. Radioactivity was converted to drug concentration based on the specific activity of the compound.
RT-PCR and Sequencing Analysis
SV40-HCEC, MDCKII-BCRP, and HGF-1 cells were grown for mRNA extraction. RT-PCR was performed based on a protocol published from our laboratory and Sugarawa et al. (2000).2,30 BCRP primers were designed from human BCRP cDNA (Genbank Accession No: AY289766). The forward and reverse primers designed for BCRP were 5 TTATCCGTGGTGTGTCTGGA 3′ and 3′ CCTGCTTGGAAGGCTCTAG 5′, respectively. RT-PCR was performed with a kit (SuperScript™III First-Strand Synthesis System for RT-PCR; Invitrogen, Carlsbad, CA, USA). Reverse transcription conditions were template RNA denaturation at 70°C for 10 min and reverse transcription at 42°C for 60 min. PCR amplification conditions were denaturation at 94°C, annealing at 58.5°C, and extension at 72°C for 1 min each for 35 cycles, followed by a final extension at 72°C for 10 min. 2% agarose gel was employed to analyze RT-PCR products. Sequencing analysis was performed by Agencourt Biosciences (Beverly, MA, USA) using Quicklane®sequencing technology.
Western Blot
A published protocol from our laboratory by Dey et al. (2003) was employed with slight modifications.2 MDCKII-BCRP cells and SV40-HCEC cultured on T-75 flasks and harvested into 5 ml of phosphate buffered saline. A Mem-PER Eukaryotic Membrane Protein Extraction Reagent Kit (Pierce Biotechnology, Inc., Rockford, IL, USA) was employed to separate the membrane fraction of the cells. The fraction was stored at – 80°C. SDS polyacrylamide gel electrophoresis (8% Trisglycine gels for 2 hr at 120 V) was used to separate the proteins. Proteins were transferred onto nitrocellulose membranes by exposing the gel for ~2 hr at ~250 mA on ice. 0.2% ponceau S (dissolved in 3% w/v trichloroacetic acid and sulfosalicylic acid)2 was used to check the protein transfer efficiency. The blot was incubated in blocking buffer (1% w/v BSA and 5% nonfat dry milk in Tris-buffered saline)2 at room temperature for 1 hr. The blot was treated with BCRP antibody (1:100) for 12 hr at 4°C. The membrane was washed 4–5 times at 5-min intervals with TBST (Tris-buffered saline + 0.1% Tween 20)2 and later treated with a secondary antibody (1:1500 anti-rat IgG-horseradish peroxidase [HRP]). The blot was washed with TBST three times each at 10-min intervals. An amplified Opti-4CN Western Blot amplification kit (Bio-Rad, Herculus, CA, USA) was used to develop the blots, according to the manufacturer's protocol.
Statistical Analysis
Experiments were performed with n = 4 (n = 3 for data in Fig. 6), and the results expressed as the mean ± SD. Statistical significance testing was performed with a two-factor analysis of variance (ANOVA; SPSS, ver. 12.0; SPSS Inc., Chicago, IL, USA). A difference between mean values was considered significant at p ≤ 0.05. The Fisher least-significance-difference (LSD) method was used to discriminate among significant differences between the mean values.
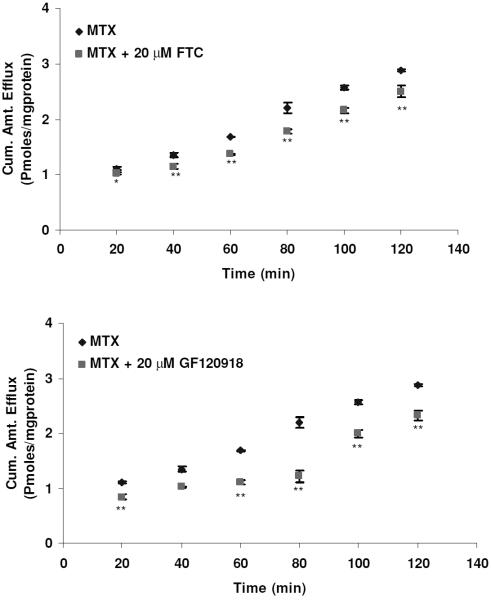
Figure 6.
Efflux of [3H]-MTX as a function of time in the presence of FTC and GF120918. Efflux was determined at different timepoints from 0 and 120 min. Each data point is representative of mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *p ≤ 0.05; **p ≤ 0.01.
RESULTS
BCRP mRNA and Protein Expression in MDCKII-BCRP and SV40-HCEC
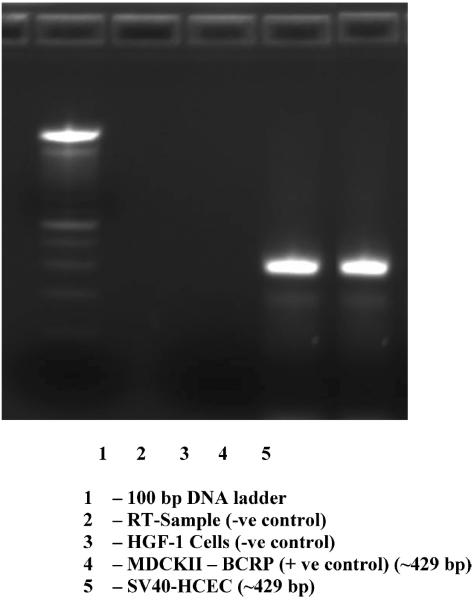
RT-PCR, conducted on cultures of human corneal epithelial cells (SV40-HCEC) and positive control cells (MDCKII-BCRP) demonstrated the presence of mRNA for BCRP (Fig. 7). The RT-PCR product sizes were correct, i.e., ~429 bp. No expression was noticed in HGF-1 cells (negative control). Sequencing, alignment in ClustalW, and comparison with the GenBank sequences showed that BCRP from SV40-HCEC was ~98% identical to the human BCRP sequence in the pubmed database. Western blot analysis using rat monoclonal BCRP antibody detected bands of the expected size in MDCKII-BCRP and SV40-HCEC, i.e., at ~70 kDa (Fig. 8).
Figure 7.
RT-PCR confirming the expression of BCRP in human corneal epithelial cells. RT-PCR has (lane 1) 100 bp DNA ladder, (lane 2) RT-sample (−ve control), (lane 3) HGF-1 cells (−ve control), (lane 4) MDCK11-BCRP (+ve control), and (lane 5) SV40-HCEC.
Figure 8.
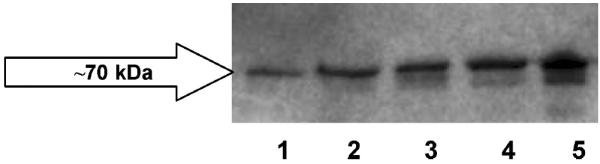
Immunoprecipitation followed by Western blot indicating the presence of BCRP in membrane fractions of human corneal epithelial cells (lanes 1, 2, and 3) and MDCK11-BCRP (positive control, lanes 4 and 5).
Efflux Studies in MDCKII-BCRP and SV40-HCEC
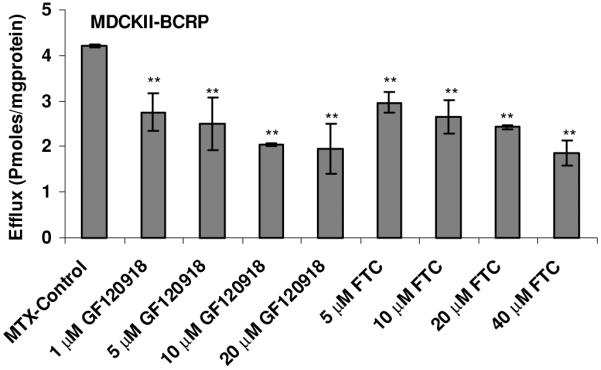
To determine whether BCRP isozymes present in SV40-HCEC were functionally active, transport studies were conducted using the organic anion [3H]-MTX. Following a 1-hr loading of 0.05 μCi/ml [3H]-MTX under ATP-depleting conditions, efflux was measured from SV40-HCEC cells by determining the concentration of [3H]-MTX effluxed into the external medium. The addition of 10 mM sodium azide and 2-deoxy-D-glucose to cell monolayers was known to decrease intracellular ATP by ~90% in the first 5 min of incubation28 and did not affect the viability of the cells, as determined by a trypan blue exclusion test. The efflux of [3H]-MTX was significantly decreased (p ≤ 0.05) in the presence of FTC and GF120918. Dose-dependent inhibition of efflux in the presence of FTC and GF120918 was observed in MDCKII-BCRP (Fig. 1). Efflux was reduced by ~40% with 1 μM GF120918, ~50% with 5 μM, and a maximum of ~60% reduction was obtained with 20 μM, respectively. Efflux was reduced by 40% with 10 μM FTC and a maximum 65% reduction ~ was obtained with 40 μM. In SV40-HCEC, a dose-dependent decrease in efflux was noticed with GF120918, and inhibition (~50%) (0.82 ± 0.17 pmoles/mg protein) was observed at a concentration of 20 μM as compared to control (1.68 ± 0.008 pmoles/mg protein) (Fig. 2). Also, a dose-dependent inhibition was observed with FTC, and a maximum of ~55% (0.79 0.01 pmoles/mg protein) inhibition was demonstrated with 40 μM FTC relative to control (1.68 ± 0.008 pmoles/mg protein) (Fig. 2). IC50 values from dose-dependent inhibition studies were ~10 μM for FTC and ~5 μM for GF120918. No relative contribution of P-gp was noticed with the use of PGP-4008, and this clearly indicates that inhibition of efflux by GF120918 was BCRP-mediated (Fig. 3). Use of 2,4-DNP, a metabolic inhibitor, showed dose-dependent suppression of ATP-mediated efflux of [3H]-MTX (Fig. 4). No pH-dependant efflux was noticed in SV40-HCEC, except at pH 5.5. Efflux was not statistically significant at pH 4.0 and 8.8 when compared to control (pH 7.4) (Fig. 5). Time-dependent efflux was observed, and the cumulative amount of efflux was statistically significant at various timepoints with ~20 μM FTC and ~20 μM GF120918 in SV40-HCEC (Fig. 6).
Figure 1.
Dose-dependent effect of FTC (A) and GF120918 (B) on the efflux of [3H]-MTX by MDCKII-BCRP cells. Monolayers of SV40-HCEC were incubated with [3H]-MTX (0.05 μCi/ml) under ATP-depleting conditions, and complete medium was added with and without inhibitor. The medium was analyzed after 1 hr. Each data point is representative of mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *p ≤ 0.05; **p ≤ 0.01.
Figure 2.
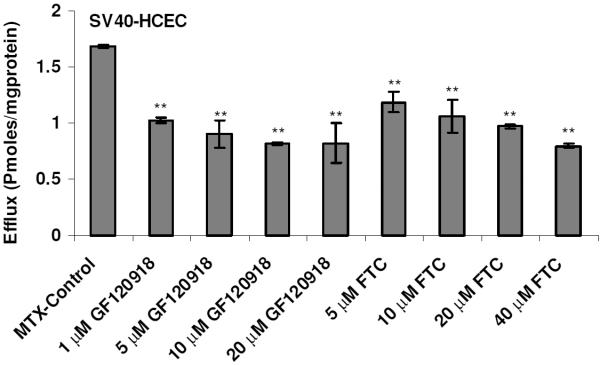
Dose-dependent effect of FTC (A) and GF120918 (B) on the efflux of [3H]-MTX by SV40-HCEC. Monolayers of SV40-HCEC were incubated with [3H]-MTX (0.05 μCi/ml) under ATP-depleting conditions, and complete medium was added with and without inhibitor. The medium was analyzed after 1 hr. Each data point is representative of mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *p ≤ 0.05; **p ≤ 0.01.
Figure 3.
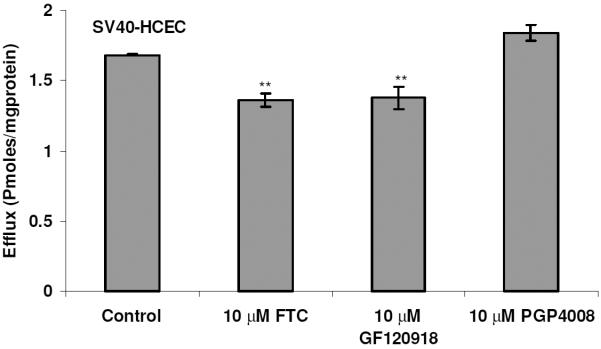
Demonstration of BCRP-specific efflux using FTC, GF120918, and PGP-4008 in SV40-HCEC. Monolayers of SV40-HCEC were incubated with [3H]-MTX (0.05 μCi/ml) under ATP-depleting conditions, and complete medium was added with and without inhibitor. The medium was analyzed after 1 hr. Each data point is representative of mean ± SD of three determinations. Statistical significance was measured by two-factor ANOVA. *p ≤ 0.05; **p ≤ 0.01.
Figure 4.
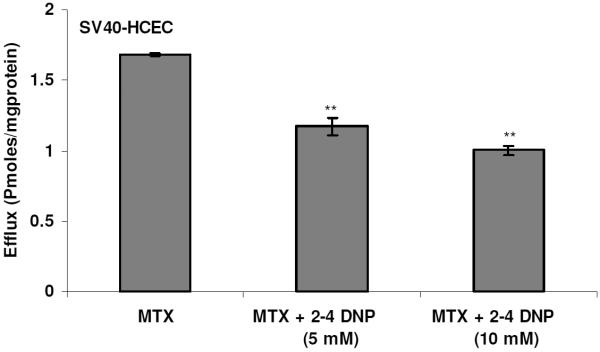
Dose-dependent inhibition of BCRP using a metabolic inhibitor 2,4-DNP. Monolayers of SV40-HCEC were incubated with [3H]-MTX (0.05 μCi/ml) and complete medium was added with and without metabolic inhibitor. The medium was analyzed after 1 hr. Each data point is representative of mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *p ≤ 0.05; **p ≤ 0.01.
Figure 5.
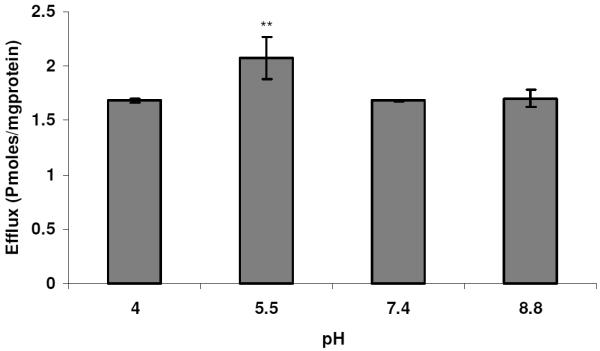
Efflux of MTX as a function of pH. Monolayers of SV40-HCEC were incubated with [3H]-MTX (0.5 μCi/ml) under ATP-depleting conditions, and complete medium with different pH ranges was added and the efflux was determined after 1 hr. Each data point is representative of mean ± SD of three determinations. Statistical significance was determined by two-factor ANOVA. *p ≤ 0.05; **p ≤ 0.01.
DISCUSSION
In this article, we are reporting the functional expression of BCRP in human corneal epithelial cells. We have demonstrated that it does play a role in drug efflux. Significant efflux of [3H]-MTX in the presence of FTC and GF120918 established the functional activity of BCRP in SV40-HCEC and its possible role in reducing the ocular bioavailability of topically applied drugs.
Dose-dependent inhibition of efflux was noticed with both FTC and GF120918 in MDCKII-BCRP (+ve control) and SV40-HCEC. Inhibition of efflux was more prominent in MDCKII-BCRP than in SV40-HCEC with the same concentrations of inhibitors. This may potentially be due to the overexpression of the BCRP transporter in the transfected cell line. FTC is a specific inhibitor of BCRP16 and does not react with P-gp/MRP, and, hence, the inhibition of efflux was representative of BCRP-mediated efflux. However, as GF120918 modulates both BCRP and P-gp,16 PGP-4008 was employed as a control, and our data indicates no role of P-gp in the transport of [3H]-MTX. This confirms that inhibition of efflux by GF120918 was due to inhibition of BCRP. This further confirms the presence of BCRP and its functional role in SV40-HCEC. Use of 2,4-DNP, a potent metabolic inhibitor,48 suggests ATP-mediated efflux of [3H]-MTX. As BCRP is a major class of ATP-binding cassette transporter proteins requiring ATP for their function, the data further supports the presence of BCRP.21 No pH-dependant efflux was noticed except at pH 5.5. It appears that high acidic conditions inactivate the transporter. Studies by Breedveld et al. (2006) indicated that BCRP was activated as pH was decreased from 7.9 to 5.1. Our results indicate that BCRP is activated at pH 5.5, but no significant activation is noticed at an acidic pH of 4.0. We do not know the reason for this discrepancy. It was indicated by Acheampong et al. (2002) that formulation factors, such as pH, preservative, and vehicle, might influence the ocular penetration of drugs.42 Though a pH of ~5.5 was shown to activate BCRP in HCEC, its role in modulating ocular penetration of drugs needs to be further investigated. Based on the nature of the active ingredient, ocular drugs are formulated at lower pH to increase aqueous solubility and stability.42 Taking buffer capacity of the pre-corneal fluids, and our results demonstrating a mild activation of BCRP at acidic pH into consideration, the role of formulation pH in activating BCRP and leading to poor permeation of drugs might be minimal.
Data in Figure 5A and 5B indicate inhibition of efflux over a period of time (120 min), and the inhibition was statistically significant at all the timepoints with the use of both FTC and GF120918. This data further indicates that BCRP is indeed functionally active in efflux of molecules across corneal epithelium and might play a significant role in ocular drug efflux. However, the relative rate of efflux can be governed by various factors, including substrate specificity of the efflux transporter and extent of expression of the transporter compared to other efflux pumps, etc. Limited data is available on substrate specificities of various ocular drugs. Therefore, additional studies are being performed to delineate the relative contributions of various efflux pumps in ocular drug efflux. RT-PCR data, combined with excellent sequence homology (98%) with human BCRP gene sequence, supports our results. Immunoprecipitation, followed by Western blot analysis, confirms such expression. Lung resistance protein (LRP) has also been identified in HCEC (unpublished data). The presence of LRP in human cornea was shown recently.43 However, to the best of our knowledge, there were no reports indicating the interaction of FTC and GF120918 with LRP, and both were found not to interact with MRP. They were used to specifically modulate BCRP. The relative significance of LRP in corneal drug resistance is undergoing further evaluation in our laboratory.
Ocular drug delivery, followed by topical administration of drugs, is not an efficient process.2 Many topically instilled drugs reach the internal segments of the eye through the cornea.2 As stated by Dey et al. (2003),2“these drugs include steroids,31,32 ß-blockers,33,34antibiotics,35,36,37 and nonsteroidal anti-inflammatory agents.38” The cul-de-sac, as a route for topical drug administration, is the most common route of ocular drug delivery.2 Absorption from the cul-de-sac involves both non-corneal (sclera and conjunctiva) and corneal routes.2 As the drug is absorbed by the non-scleral route, the drug is eventually eliminated through the general circulation.2 This results in poor ocular bioavailability.2 From the corneal route, lipoidal corneal epithelium, tight junctions, and efflux transporters (such as Pgp and MRP) offer significant resistance.2,3,4 Solution drainage and lacrimation include other modes of drug elimination.2
Our studies indicate that BCRP may cause the efflux of molecules out of corneal epithelium into the precorneal fluid and may contribute as one of the factors leading to ocular drug resistance. BCRP was found to be expressed at a relatively low level in stem cells, which helps in regenerating corneal epithelium.21 Hence, it is regarded as a putative stem cell marker used to identify the corneal stem cells responsible for regeneration of epithelial cells. This is of high clinical significance, as constructs generated from limbal epithelial cells were successfully used in clinical transplantation to human patients.23 The fact that BCRP was found on stem cells and on human corneal epithelial cells, and demonstrated rapid efflux of substrate molecules, provides new insight into ocular drug resistance. Relative expression levels of BCRP between stem cells and epithelial cells can be investigated in the future, which might provide a new approach to ocular drug delivery. Ocular pharmacokinetic studies by Dey et al. (2004) and Karla et al. (2008) indicate that efflux pumps do indeed pose a major barrier to ocular drug delivery.39,40,41 MRPs (MRP1, MRP2, and MRP3) were found to cause significant resistance to the absorption of erythromycin following topical administration in male New Zealand white rabbits.41 When MK571, a specific inhibitor of MRP, was employed, Cmax and AUC0--∞ were (0.40 ± 0.05 μg/ml) and (67.9 ± 3.81 μg/min/ml) compared to the corresponding values for control Cmax (0.13 ± 0.01 μg/ml) and AUC0--∞ (3.17 ± 4.65 μg/min/ml)41. The significant increase in aqueous humor concentrations demonstrated functional activity of MRP in vivo.42 As indicated in our earlier publication, Karla et al. (2007), efflux transporters play a collective role in conferring resistance to the absorption of common substrates.3 As indicated in the publication, erythromycin is a substrate for both Pgp and MRP.3 Both the transporters were found to play a collective role in the efflux of erythromycin.3 In light of new efflux transporters like BCRP, ocular drugs need to be screened for their relative substrate specificities with the transporters. A drug that is a common substrate for Pgp, MRP, and BCRP can encounter a significant resistance to the permeation across the cornea as the three transporters might act as a collective barrier preventing drug absorption. A diagrammatic representation of BCRP embedded in the lipid bilayer of corneal epithelial cell leading to drug efflux is shown in Figure 9. A detailed structure of BCRP was also shown. In the light of new efflux transporters like BCRP, further studies are being conducted in our laboratory to study the relative role of efflux pumps to alter the ocular pharmacokinetics of various drugs.
Figure 9.
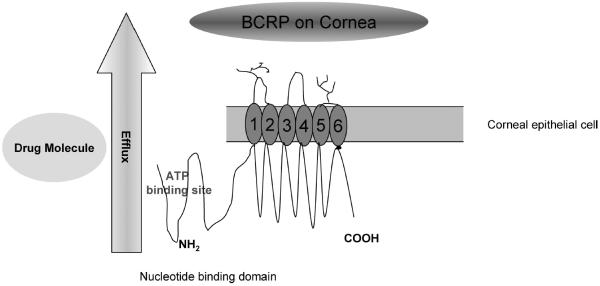
Pictorial representation for the presence of BCRP on human corneal epithelial cell leading to drug efflux. BCRP is a half transporter having six transmembrane domains and one ATP-binding domain. Figure drawn based on inspiration from Perez-Plasencia et al. (2006)46 and Gottesman et al. (2002)46.
ACKNOWLEDGMENTS
We sincerely thank Dr. Thomas Johnston, UMKC School of Pharmacy, for his generous contribution with HGF1 cells. We thank Dr. Swapan Samanta, UMKC School of Pharmacy, and Dr. Priscilla Thomas, UMKC School of Medicine, for their help in transport studies. We acknowledge NIH Grants R01 EY09171-12, 13 and R01 EY10659-10 for the support.
Footnotes
After we disclosed our findings for the presence of BCRP in the current manuscript submitted on October 2006, we submitted the final revision for acceptance in September 2008. The significant delay was due to a time lapse in procuring a batch of SV40-HCEC which does not employ cholera toxin. We came across two additional findings for the presence of BCRP in human corneal cells and tissue and these were published after our initial submission.43,44
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Copyright of Current Eye Research is the property of Taylor & Francis Ltd and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
REFERENCES
- 1.Ito K, Oleschuk CJ, Westlake C, Vasa MZ, Deeley RG, Cole SP. Mutation of Trp1254 in the multispecific organic anion transporter, multidrug resistance protein 2 (MRP2) (ABCC2), alters substrate specificity and results in loss of methotrexate transport activity. J Biol Chem. 2001;41:38108–38114. doi: 10.1074/jbc.M105160200. [DOI] [PubMed] [Google Scholar]
- 2.Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, Kaliki P, Pal D, Ganapathy V, Mitra AK. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 3.Karla PK, Pal D, Mitra AK. Molecular evidence and functional expression of multidrug resistance associated protein (MRP) in rabbit corneal epithelial cells. Exp Eye Res. 2007;84:53–60. doi: 10.1016/j.exer.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Karla PK, Pal D, Quinn T, Mitra AK. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int J Pharm. 2007;336:12–21. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karla PK, Pal D, Mitra AK. Molecular evidence and functional expression of multi-drug resistance associated protein (MRP) in human corneal epithelium, rabbit cornea, and evaluation of its role using a specific inhibitor (AAPS abstract) 2006. [Google Scholar]
- 6.Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, Suzuki N, Tsuji A. Involvement of multidrug resistance-associated protein 2 in intestinal secretion of grepafloxacin in rats. Antimicrob Agents Chemother. 2002;46:344–349. doi: 10.1128/AAC.46.2.344-349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seral C, Carryn S, Tulkens PM, Van Bambeke F. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J Antimicrob Chemother. 2003;51:1167–1173. doi: 10.1093/jac/dkg223. [DOI] [PubMed] [Google Scholar]
- 8.Michot JM, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Active efflux of ciprofloxacin from J774 macrophages through an MRP-like transporter. Antimicrob Agents Chemother. 2004;48:2673–2682. doi: 10.1128/AAC.48.7.2673-2682.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ZS, Guo Y, Belinsky MG, Kotova E, Kruh GD. Transport of bile acids, sulfated steroids, estradiol 17-beta-D-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11) Mol Pharmacol. 2005;67:545–557. doi: 10.1124/mol.104.007138. [DOI] [PubMed] [Google Scholar]
- 10.Chu XY, Huskey SE, Braun MP, Sarkadi B, Evans DC, Evers R. Transport of ethinylestradiol glucuronide and ethinylestradiol sulfate by the multidrug resistance proteins MRP1, MRP2, and MRP3. J Pharmacol Exp Ther. 2004;309:156–164. doi: 10.1124/jpet.103.062091. [DOI] [PubMed] [Google Scholar]
- 11.Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, Borst P. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4) Biochem J. 2003;371:361–367. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bredel M, Bredel C, Sikic BI. Genomics-based hypothesis generation: A novel approach to unraveling drug resistance in brain tumors. Lancet Oncol. 2004;5:89–100. doi: 10.1016/S1470-2045(04)01382-8. [DOI] [PubMed] [Google Scholar]
- 13.Sawicka M, Kalinowska M, Skierski J, Lewandowski W. A review of selected anti-tumor therapeutic agents and reasons for multidrug resistance occurance. J Pharm Pharmacol. 2004;56:1067–1081. doi: 10.1211/0022357044265. [DOI] [PubMed] [Google Scholar]
- 14.Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyake K, Mickley L, Litman T, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 16.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 17.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;7:E118–133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klucken J, Buchler C, Orso E. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci USA. 2000;97:817–822. doi: 10.1073/pnas.97.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graf GA, Yu L, Li WP, et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half ABC transporter ABCG2/BCRP/MXR/ABCP in plasma membranes. J Biol Chem. 2004;279:19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem. 2003;278:22644–22649. doi: 10.1074/jbc.M212399200. [DOI] [PubMed] [Google Scholar]
- 22.Imai Y, Asada S, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol. 2003;64:610–618. doi: 10.1124/mol.64.3.610. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z-S, Robey RW, Belinsky MG, et al. Transport of methotrexate, methotrexate polyglutamates, and 17 β-estradiol 17-(β-D-glucuronide) by ABCG2: Effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63:4048–4054. [PubMed] [Google Scholar]
- 24.Umemoto T, Yamato M, Nishida K, Kohno C, Yang J, Tano Y, Okano T. Rat limbal epithelial side population cells exhibit a distinct expression of stem cell markers that are lacking in side population cells from the central cornea. FEBS Lett. 2005;579:6569–6574. doi: 10.1016/j.febslet.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 25.Arne H, Hana T, Lotta S, Hannu U. Evaluation of adverse ocular effects of 5-fluorouracil by using human corneal epithelial cell cultures. J of Tox. 2002;21:283–292. [Google Scholar]
- 26.Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 27.Breedveld P, Pluim D, Cipriani G, Dahlhaus F, van Eijndhoven MA, de Wolf CJ, Kuil A, Beijnen JH, Scheffer GL, Jansen G, Borst P, Schellens JH. The effect of low pH on breast cancer resistance protein (ABCG2)-mediated transport of methotrexate, 7-hydroxymethotrexate, methotrexate diglutamate, folic acid, mitoxantrone, topotecan, and resveratrol in in vitro drug transport models. Mol Pharmacol. 2007;71:240–249. doi: 10.1124/mol.106.028167. [DOI] [PubMed] [Google Scholar]
- 28.Sikri V, Pal D, Jain R, Kalyani D, Mitra AK. Cotransport of macrolide and fluoroquinolones, a beneficial interaction reversing P-glycoprotein efflux. Am J Ther. 2004;11:433–442. doi: 10.1097/01.mjt.0000132643.69143.64. [DOI] [PubMed] [Google Scholar]
- 29.Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, Kumar A, Fridland A. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048–1051. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 30.Dallas S, Zhu X, Baruchel S, Schlichter L, Bendayan R. Functional expression of the multidrug resistance protein 1 in microglia. J Pharmacol Exp Ther. 2003;307:282–290. doi: 10.1124/jpet.103.054304. [DOI] [PubMed] [Google Scholar]
- 31.Sugawara M, Nakanishi T, Fei YJ, Huang W, Ganapathy ME, Leibach FH, Ganapathy V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J Biol Chem. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- 32.Kupferman A, Leibowitz HM. Topically applied steroids in corneal disease. IV. The role of drug concentration in stromal absorption of prednisolone acetate. Arch Ophthalmol. 1974;91:377–380. doi: 10.1001/archopht.1974.03900060389009. [DOI] [PubMed] [Google Scholar]
- 33.Leibowitz HM, Kupferman A. Anti-inflammatory effectiveness in the cornea of topically administered prednisolone. Invest Ophthalmol. 1974;13:757–763. [PubMed] [Google Scholar]
- 34.Huang HS, Schoenwald RD, Lach JL. Corneal penetration behavior of beta-blocking agents III: In vitro/in vivo correlations. J Pharm Sci. 1983;72:1279–1281. doi: 10.1002/jps.2600721110. [DOI] [PubMed] [Google Scholar]
- 35.Schoenwald RD, Huang HS. Corneal penetration behavior of beta-blocking agents I: Physiochemical factors. J Pharm Sci. 1983;72:1266–1272. doi: 10.1002/jps.2600721108. [DOI] [PubMed] [Google Scholar]
- 36.Barza M, Kane A, Baum J. Pharmacokinetics of intravitreal carbenicillin, cefazolin, and gentamicin in rhesus monkeys. Invest Ophthalmol Vis Sci. 1983;24:1602–1606. [PubMed] [Google Scholar]
- 37.Mayhew JW, Fiore C, Murray T, Barza M. An internally standardized assay for amphotericin B in tissues and plasma. J Chromatogr. 1983;274:271–279. doi: 10.1016/s0378-4347(00)84430-8. [DOI] [PubMed] [Google Scholar]
- 38.Baum J, Barza M. Topical vs. subconjunctival treatment of bacterial corneal ulcers. Ophthalmology. 1983;90:162–168. doi: 10.1016/s0161-6420(83)34583-8. [DOI] [PubMed] [Google Scholar]
- 39.Agata M, Tanaka M, Nakajima A, Fujii A, Kuboyama N, Tamura T, Araie M. Ocular penetration of topical diclofenac sodium, a non-steroidal anti-inflammatory drug, in rabbit eye. Nippon Ganka Gakkai Zasshi. 1984;88:991–996. [PubMed] [Google Scholar]
- 40.Dey S, Gunda S, Mitra AK. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: Role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J Pharmacol Exp Ther. 2004;311:246–255. doi: 10.1124/jpet.104.069583. [DOI] [PubMed] [Google Scholar]
- 41.Karla PK, Quinn TL, Betty HL, Thomas P, Pal D, Mitra AK. AAPS abstract. 2008. Pharmacokinetic evaluation of a nucleotide efflux transporter on cornea. [Google Scholar]
- 42.Karla PK, Quinn TL, Samanta SK, Mitra AK. AAPS abstract. 2007. A specific inhibitor can overcome nucleotide efflux transporter on human and rabbit corneal epithelial cells and significantly increase anterior segment drug delivery. [Google Scholar]
- 43.Acheampong AA, Small D, Baumgarten V, Welty D, Tang-Liu D. Formulation effects on ocular absorption of brimonidine in rabbit eyes. J Ocul Pharmacol Ther. 2002;18:325–337. doi: 10.1089/10807680260218498. [DOI] [PubMed] [Google Scholar]
- 44.Becker U, Ehrhardt C, Daum N, Baldes C, Schaefer UF, Ruprecht KW, Kim KJ, Lehr CM. Expression of ABC-transporters in human corneal tissue and the transformed cell line, HCE-T. J Ocul Pharmacol Ther. 2007;23:172–181. doi: 10.1089/jop.2006.0095. [DOI] [PubMed] [Google Scholar]
- 45.Zhang T, Xiang CD, Gale D, Carreiro S, Wu EY, Zhang EY. Drug transporter and cytochrome P450 mRNA expression in human ocular barriers: Implications for ocular drug disposition. Drug Metab Dispos. 2008;36:1300–1307. doi: 10.1124/dmd.108.021121. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Plasencia C, Duenas-Gonzalez A. Can the state of cancer chemotherapy resistance be reverted by epigenetic therapy? Mol Cancer. 2006;5:27. doi: 10.1186/1476-4598-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 48.Lee BD, French KJ, Zhuang Y, Smith CD. Development of a syngeneic in vivo tumor model and its use in evaluating a novel P-glycoprotein modulator, PGP-4008. Oncol Res. 2003;14:49–60. doi: 10.3727/000000003108748603. [DOI] [PubMed] [Google Scholar]
- 49.Fry DW, White JC, Goldman ID. Effects of 2,4-dinitrophenol and other metabolic inhibitors on the bidirectional carrier fluxes, net transport, and intracellular binding of methotrexate in Ehrlich ascites tumor cells. Cancer Res. 1980;40(10):3669–3673. [PubMed] [Google Scholar]