Abstract
A technique is presented for the isolation of vacuoles from Sedum telephium L. leaves. Leaf material is digested enzymically to produce protoplasts rapidly which are partially lysed by gentle osmotic shock and the inclusion of 5 millimolar ethyleneglycol-bis (β-aminoethyl ether)N,N′-tetraacetic acid in the wash medium. Vacuoles are isolated from the partially lysed protoplasts by brief centrifugation on a three-step Ficoll-400 gradient consisting of 5, 10, and 15% (w/v) Ficoll-400. A majority of the vacuoles accumulate at the 5 to 10% Ficoll interface, whereas a smaller proportion sediments at the 10 to 15% Ficoll-400 interface. The total time required for vacuole isolation is 2 to 2.5 hours, beginning from leaf harvest.
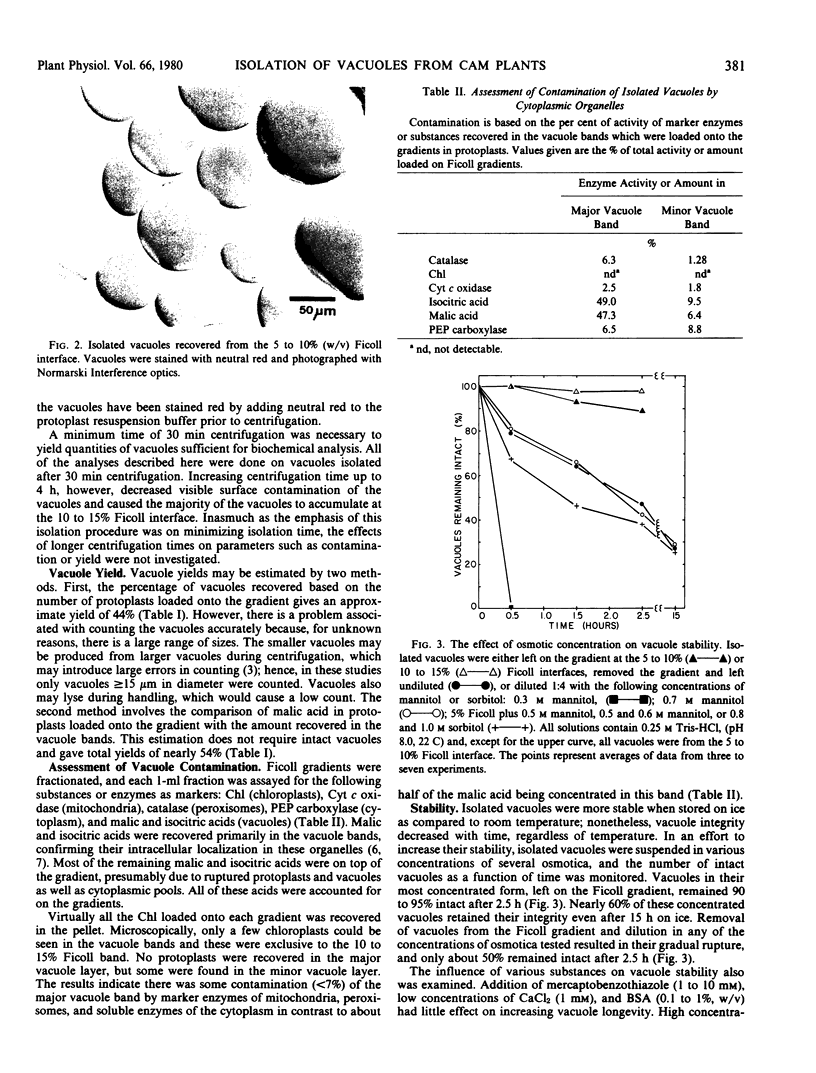
The yield of vacuoles is approximately 44%. The major vacuole layer is < 7% contaminated by marker enzymes from the cytoplasm and other organelles but shows no contamination by chloroplasts. Isolated vacuoles were stable for >15 hours when left in Ficoll; however, dispersion into media of various osmotic concentrations resulted in decreased stability. Addition of mercaptobenzothiazole, CaCl2, MgCl2, bovine serum albumin, ethylenediaminetetraacetic acid, polyethylene glycol 600, and KH2PO4 to the vacuole isolation media did not increase the stability of the isolated vacuoles.
This technique with only slight modifications has been used to isolate leaf cell vacuoles from the following Crassulacean acid metabolism plants: pineapple, Kalanchoë fedtschenkoi, and Echeveria elegans. Spinach leaves also were used successfully.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. A., Rees T., Fuller W. A., Banfield J. The location of acid invertase activity and sucrose in the vacuoles of storage roots of beetroot (Beta vulgaris). Biochem J. 1979 Mar 15;178(3):539–547. doi: 10.1042/bj1780539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji S. K., Ting I. P. Phosphoenolpyruvate carboxylase isoenzymes: separation and properties of three forms from cotton leaf tissue. Arch Biochem Biophys. 1971 Mar;143(1):297–317. doi: 10.1016/0003-9861(71)90212-8. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Hydrolases in vacuoles from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E. Presence of the cyanogenic glucoside dhurrin in isolated vacuoles from sorghum. Plant Physiol. 1978 Feb;61(2):154–157. doi: 10.1104/pp.61.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A. Investigations of vacuoles isolated from tobacco: I. Quantitation of nicotine. Plant Physiol. 1979 Jul;64(1):74–78. doi: 10.1104/pp.64.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979 Jul;64(1):88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Immunological Identification of Proteinase Inhibitors I and II in Isolated Tomato Leaf Vacuoles. Plant Physiol. 1977 Jul;60(1):61–63. doi: 10.1104/pp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]




